Medical/Pharmaceuticals
Happiness Biotech Entered Into Electric Vehicle Distribution Agreement With Geely's Subsidiary
NANPING, China, June 10, 2021 /PRNewswire/ -- Happiness Biotech Group Limited (the "Company" or Nasdaq: HAPP), an innovativeChina-based nutraceutical and dietary supplements producer and e-commerce services provider, announced today that Taochejun (Fujian) Automobile Distribution Co., Ltd. ("Taoc...
OliPass Expands Scientific Advisory Board by Inviting Experts in Pain To Bolster Phase 2 Clinical Trial Design and Management
SEOUL, South Korea, June 10, 2021 /PRNewswire/ -- OliPass Corporation, a South Korea based biotech specialized in the development of RNA therapeutics, announced that it is expanding its Scientific Advisory Board ("SAB") for SCN9A antisense pain killer OLP-1002 to include ProfessorPatrick M. Do...
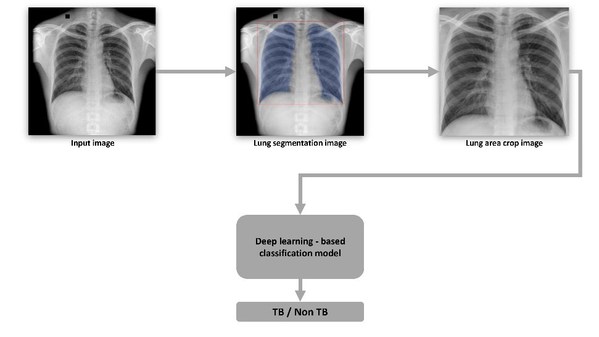
VinBrain and FIT Jointly Release a White Paper on Utilizing Artificial Intelligence in Tuberculosis Screening
HANOI, Vietnam, June 10, 2021 /PRNewswire/ -- On June 10, 2021, VinBrain (Vingroup) and FIT (a German NGO working in the field of Tuberculosis prevention and control) jointly release a white paper on utilizing artificial intelligence (AI) in Tuberculosis (TB) diagnosis and screening inVietnam. Th...
Verona Pharma and Nuance Pharma Announce $219 Million Strategic Collaboration to Develop and Commercialize Ensifentrine in Greater China
LONDON and RALEIGH, N.C., and SHANGHAI, June 10, 2021 /PRNewswire/ -- Verona Pharma plc (Nasdaq: VRNA) ("Verona Pharma") and Nuance Pharma Limited ("Nuance Pharma"), today announce that the companies have entered into an agreement granting Nuance Pharma, aShanghai-based specialty pharmaceutical c...
Enamine implements CDD Vault to digitalize its integrated medicinal chemistry, ADME-PK and screening services
The company's expansion in the biological assay space aided by the data platform SAN FRANCISCO and KYIV, Ukraine, June 10, 2021 /PRNewswire/ -- Enamine, a leading provider of R&D Services as well asScreening Compounds, Fragments, Building Blocks and specialized libraries for Drug Discovery, annou...
Peijia Medical Partners with inQB8 for US Incubator and Transcatheter Tricuspid Replacement (TTVR) Product
SUZHOU, China, June 9, 2021 /PRNewswire/ -- Peijia Medical (HKEX:9996, or " Peijia"), a leading player in China for medical technology, announced a partnership withinQB8, a Boston based medical technology incubator, to explore innovative solutions for Structural Heart Disease. This partnership inc...
WuXi XDC and LegoChem Biosciences Signed Memorandum of Understanding for the Development and Manufacturing of Antibody-drug Conjugates
WUXI, China and DAEJEON, South Korea, June 10, 2021 /PRNewswire/ -- WuXi XDC ("XDC"), a global CDMO company dedicated to end-to-end bioconjugates services, and LegoChem Biosciences (141080KS,"LCB"), today announced that a Memorandum of Understanding (MOU) was signed to form a strategic partnershi...
Canadian Medical Auctions Launches First Auction in Toronto, Canada
TORONTO, June 10, 2021 /PRNewswire/ -- Canadian Medical Auctions, dedicated to the re-marketing and auction of surplus healthcare equipment, is delighted to announce their official launch, as the first specialized medical equipment auction house inCanada. Part of the BMA Group, Canadian Medical ...
Zeus Industrial Products to Integrate Catheter-Based Contract Manufacturer CathX Medical
ORANGEBURG, S.C., June 9, 2021 /PRNewswire/ -- Zeus Industrial Products, Inc. (Zeus), the global leader in advanced polymer solutions, announced today that it has finalized an agreement to integrate CathX Medical Inc. (CathX) into its organization. Based inSan Jose, California, CathX is a medical...
Travecta Therapeutics Appoints Charles S. Ryan as President and Chief Executive Officer
SINGAPORE, June 9, 2021 /PRNewswire/ -- Travecta Therapeutics, Pte Ltd., a preclinical stage biopharmaceutical company pioneering a portfolio of product candidates engineered to cross the blood-brain-barrier, today announced the appointment ofCharles S. Ryan, JD, PhD as President and Chief Execut...
/C O R R E C T I O N -- Amorepacific/
In the news release, A green tea probiotic strain found by Amorepacific is added to the U.S. FDA's NDI list, issued08-Jun-2021 by Amorepacific over PR Newswire, we are advised by the company that the [in the second, third, fourth and fifth] paragraphs, there are few changes]" as originally issued...
China Pharma to Launch Highly Purified NMN+PQQ Product
HAIKOU, China, June 9, 2021 /PRNewswire/ -- China Pharma Holdings, Inc. (NYSE American: CPHI) ("China Pharma," the "Company" or "We"), a specialty pharmaceutical company, today announced plans to launch a highly purified NMN+PQQ product following the recent successful completion of a pilot scale ...
European Patent Office upholds Curadev's cyclodextrin patent for new composition of matter
SANDWICH, England, June 9, 2021 /PRNewswire/ -- Sulphobutyl ether β-cyclodextrin (SBE-β-CD) is used as an excipient to improve the solubility and stability of a range of active pharmaceutical ingredients, prominent among which is the antifungal drug, voriconazole. Working with ProfessorSteve Wick...
Travecta Therapeutics Appoints Charles S. Ryan as President and Chief Executive Officer
SINGAPORE, June 9, 2021 /PRNewswire/ -- Travecta Therapeutics, Pte Ltd. incorporated inSingapore on 7th April 2017, a preclinical stage biopharmaceutical company pioneering a portfolio of product candidates engineered to cross the blood-brain-barrier, today announced the appointment of Charles S....
Product Quality is of the Utmost Importance, Visitors to the ICMD Impressed by ICP DAS-BMP Medical Grade TPU
HSINCHU, June 9, 2021 /PRNewswire/ -- The 31st International Component
Manufacturing & Design Show (ICMD) took place fromMay 13 to 16, 2021 at the
Shanghai National Convention and Exhibition Center. The event was a big
success, with industry heavyweights in attendance.
LivFul and Global Access Diagnostics announce partnership for COVID-19 test kit distribution
LIVERPOOL, England, June 9, 2021 /PRNewswire/ -- LivFul and Global Access Diagnostics (GAD) today announced a new partnership to make a variety of diagnostic kits, starting with COVID-19 rapid antigen tests, available in Low to Middle Income Countries. While new innovations are being developed, ...
Antengene's Partner Karyopharm Therapeutics Announces Updated Data of Eltanexor in Patients with Hypomethylating Agent Refractory MDS
--Of the 15 patients evaluable for efficacy, 7 (47%) had mCR and 5 (33%) had SD for a total disease control rate of 80%-- --Patients with mCR had longer mOS than patients without mCR or with PD-- SHANGHAI and HONG KONG, June 9, 2021 /PRNewswire/ -- Antengene's Partner, Karyopharm Therapeutics In...
Sirtex Medical provides donations to Massachusetts Institute of Technology (MIT) and Brigham and Women's Hospital in support of the Artzi Lab, as part of Sirtex's corporate citizenship strategy to support research and innovation
WOBURN, Mass., June 8, 2021 /PRNewswire/ -- Sirtex Medical (Sirtex), a leading manufacturer of targeted liver cancer therapies, has provided grants to Massachusetts Institute of Technology (MIT) and Brigham and Women's Hospital for the advancement of cancer treatment, to be shared with the indepen...
Innoforce Pharmaceuticals to Participate in BIO International Digital Convention
HANGZHOU, China and ROCKVILLE, Md., June 8, 2021 /PRNewswire/ -- Innoforce Pharmaceuticals ("Innoforce"), an innovation and partnership-focused biopharmaceutical company, today announces that the company is participating in the BIO International Digital Convention (BIO Digital) being held fromJun...
Neoss Announces NeossONE™ - One Platform, Smart Prosthetics
NeossONE™ is a solution unique to the Neoss® Implant System - one prosthetic platform, across three implant ranges, including ALL implant diameters and abutments. Simply put, the same prosthetic components fit every implant.¹ HARROGATE, England, June 8, 2021 /PRNewswire/ -- ONE Prosthetic Platfo...
Week's Top Stories
Most Reposted
DBS is First Bank in Asia Pacific to Pilot Visa Intelligent Commerce for Everyday Payments
[Picked up by 319 media titles]
2026-02-16 10:00Marina Bay precinct partners UOB, Marina Bay Sands and Singapore Tourism Board, together with Disney Cruise Line, to illuminate Singapore's skyline with a fireworks sky show
[Picked up by 316 media titles]
2026-02-19 14:30Little Artists Art Studio, Singapore Shines at Art Capital 2026
[Picked up by 277 media titles]
2026-02-17 19:12Tower Capital Asia announces majority investment in V-Key - a leader in digital identity and mobile application security
[Picked up by 265 media titles]
2026-02-13 01:52Appier Delivers Record Results Driven by Agentic AI Innovation
[Picked up by 263 media titles]
2026-02-13 17:03