Medical/Pharmaceuticals
Neurophet to present brain stimulation simulation study at OHBM 2024
- Presents a poster identifying tDCS effects for patients with AD and cognitively normal people - Showcases brain imaging treatment planning software for electric brain stimulation Neurophet tESplan Plus SEOUL, South Korea, June 26, 2024 /PRNewswire/ -- Neurophet, an artificial intelligence (AI...
Positive Xanamem® biomarker trial published in the Journal of Alzheimer's Disease demonstrating potential Xanamem efficacy in patients with elevated blood pTau
The prospectively defined, double-blind analysis of biomarker-positive patients with mild Alzheimer's disease showed more rapid clinical progression in biomarker-positive patients, highlighting the suitability of elevated pTau for selecting patients in the on-going XanaMIA phase2b trial SYDNEY, ...
CARING PHARMACY UNVEILS MOM & CUTIE PRODUCT LINE IN CELEBRATORY 30TH ANNIVERSARY EVENT
KUALA LUMPUR, Malaysia, June 26, 2024 /PRNewswire/ -- CARiNG Pharmacy proudly celebrates its 30th anniversary with the grand launch of their 'Mom and Cutie' product line. To commemorate this milestone event, CARiNG Pharmacy held a Health & Beauty Carnival at 1 Utama Shopping Centre, Ground Floor ...
Fufang E'jiao Syrup's Breakthrough Research on Cancer-Related Fatigue Receives "Special Excellence Award" at 2024 ASCO Annual Meeting
CHICAGO, June 26, 2024 /PRNewswire/ -- The 2024 Annual Meeting of the American Society of Clinical Oncology (ASCO), a significant event in the global oncology community, was grandly held inChicago, USA, from May 31 to June 4, 2024. ASCO has announced the list of outstanding abstracts for 2024, wh...
Antengene Announces XPOVIO® (selinexor) National Health Insurance Service Approval for Reimbursement in South Korea
- XPOVIO® is the first XPO1 inhibitor approved for reimbursement by South Korea's National Health Insurance Service (NHIS) for the treatment of adult patients with relapsed/refractory multiple myeloma (R/R MM). - The approval of XPOVIO® by the NHIS in South Korea is the fourth national reimburse...
Apollo Cancer Centres Collaborates With Accuray to Launch India Sub- Continent's First Robotic Stereotactic Radiotherapy Program
* The program will enhance the development of oncology care, thereby, advancing healthcare landscape of the region * Participants will engage in in-depth discussions and demonstrations, ensuring a robust and practical learning experience BANGALORE, India, June 26, 2024 /PRNewswire/ -- Apollo...
Simcere Zaiming Announce Approval of Cetuximab Beta in China by the NMPA
NANJING, China, June 26, 2024 /PRNewswire/ -- On June 25, 2024, Simcere Zaiming, an innovative oncology company and a subsidiary of Simcere Pharmaceutical Group (2096.HK), announced that Enlituo® (generic name: cetuximab beta injection), a new generation anti-epidermal growth factor receptor (EG...
Hyundai Bioscience succeeds in developing 'Multi-treatment for mosquito-borne viral infections' including Dengue Fever
* Developed a 'Niclosamide-based multi-treatment drug' that can simultaneously treat mosquito-borne viral infections such as four serotypes of dengue virus, Zika, Chikungunya, and Yellow Fever. * Accelerating preparations for dengue fever basket clinical trial scheduled to be conducted inBraz...
Yoshihiro Yoneda Appointed President of the International Human Frontier Science Program Organization
STRASBOURG, France, June 26, 2024 /PRNewswire/ -- The International Human Frontier Science Program Organization (HFSPO) is pleased to announce that acclaimed Japanese cell biologist and international research leaderYoshihiro Yoneda will assume the role of President for the global life science or...
ONO PHARMA USA Announces Support for Conquer Cancer®, the ASCO Foundation
Conquer Cancer Supports Cancer Research and Education CAMBRIDGE, Mass., June 25, 2024 /PRNewswire/ -- ONO PHARMA USA today announced it will provide a sponsorship to Conquer Cancer®, the ASCO Foundation, with$1 million funding to advance cancer research and education. Conquer Cancer is a glob...
Endogenex™ Announces $88 Million Series C Financing to Complete Pivotal Trial of the ReCET™ System in Patients with Type 2 Diabetes
Oversubscribed financing led by strategic investor, with participation from new and existing investors MINNEAPOLIS, June 25, 2024 /PRNewswire/ -- Endogenex, a clinical-stage medical device company dedicated to improving outcomes in individuals with type 2 diabetes, announced it has closed an ove...
Porton Advanced introduces the MaxCyte ExPERT GTx Flow Electroporation instrument, continuing to provide customers with end-to-end cell and gene therapy CRO & CDMO services
SUZHOU, China, June 25, 2024 /PRNewswire/ -- Porton Advanced recently introduced the MaxCyte cGMP-grade ExPERT GTx Flow Electroporation instrument to its cell therapy platform, marking the company as the first cell therapy CDMO in China to possess this clinical-grade flow electroporation system. T...
Metabolon Receives NIH Grant to Develop Advanced Monitoring and Predictive Tools for Type 1 Diabetes Progression
MORRISVILLE, N.C., June 25, 2024 /PRNewswire/ -- Metabolon, Inc., the global leader in providing metabolomics solutions advancing a wide variety of life science research, diagnostic, therapeutic development, and precision medicine applications, is proud to announce the receipt of a prestigious gr...
CLL Community Urges Hospital Authority to List Next-Generation BTK Inhibitor on Drug Formulary to Bring Hope and Improve Quality of Life for CLL Patients
HONG KONG, June 25, 2024 /PRNewswire/ -- Chronic Lymphocytic Leukaemia ('CLL') is a lymphoproliferative disease characterised by the abnormal proliferation of lymphocytes. The progression of CLL is relatively slow and it is a mild haematological malignancy. Cypress Charitable Trust is aHong Kong ...
NX Group Signs Strategic Partnership Agreement with Controlant of Iceland
- Set to Provide Real-time Monitoring Service for Tracking Cargo Location and Strict Temperature Control - TOKYO, June 25, 2024 /PRNewswire/ -- NIPPON EXPRESS HOLDINGS, INC. has signed a strategic partnership agreement withIceland-based Controlant, Inc., a leading provider of real-time monitorin...
Singclean: Unveiled Singfiller® and Singderm® in IMCAS ASIA 2024, Poised for Thailand's Big Launch
BANGKOK, June 25, 2024 /PRNewswire/ -- Singclean, a global player and a leading Chinese manufacturer of high-quality medical aesthetic products, was excited to announce in IMCAS Asia that Singfiller® and Singderm® HA fillers will launch in Thailand this November. Although still not officially cer...
Innovent Presents the Results of the First Phase 3 Study of Mazdutide for Weight Management at the ADA's 84th Scientific Sessions
SAN FRANCISCO and SUZHOU, China, June 25, 2024 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, autoimmune, cardiovascular and metabolic, ...
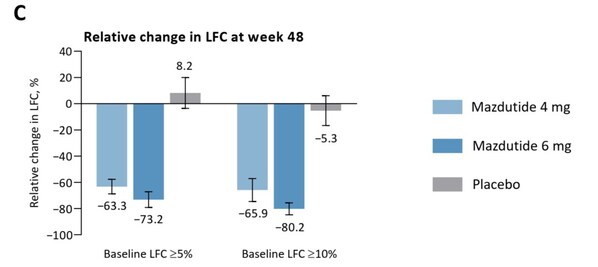
Innovent Announces Mazdutide Demonstrates 80.2% Reduction in Liver Fat Content in Exploratory Analysis of Phase 3 Weight Management GLORY-1 Study at ADA 2024
SAN FRANCISCO and SUZHOU, China, June 25, 2024 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, autoimmune, cardiovascular and metabolic, ...
NABR Commends the IUCN's Decision to Reassess the Status of Long-Tailed Macaques
A Science-Based Approach Will Determine if the Species is Truly 'Endangered' WASHINGTON, June 25, 2024 /PRNewswire/ -- The National Association for Biomedical Research (NABR) commends the Union for the Conservation of Nature (IUCN)'s decision to reassess the 'Endangered' designation of long-taile...
TiumBio Presents Promising Clinical Data from Phase 1 of its Hemophilia Treatment Candidate TU7710 at ISTH 2024
* TU7710, long-acting recombinant activated factor VII, demonstrated a 5 to 7 times longer half-lifein a Phase 1 study than that of NovoSeven, a conventional hemophilia treatment for patients who develop inhibitors * TiumBio presented interim Phase 1a clinical data of TU7710 and discussed pot...
Week's Top Stories
Most Reposted
Visa partners with Laufey to spread the magic of travel in Asia Pacific; to be Official Payment Partner for Laufey: A Matter of Time Tour
[Picked up by 309 media titles]
2026-03-04 12:35SMU and Fudan Launch Region's First Tech-Focused DBA
[Picked up by 300 media titles]
2026-03-02 09:15Infobip is set to launch AgentOS to orchestrate autonomous AI-driven customer journeys at scale
[Picked up by 290 media titles]
2026-03-02 09:00Klook's Spring Readiness Index shows how Asia's travelers are preparing for spring travel across Japan, South Korea, and Mainland China
[Picked up by 290 media titles]
2026-03-03 15:49COL and NASDAQ-Listed BeLive Holdings Unveil World's First "Microdrama in a Box" in Headline Hong Kong FILMART 2026 Launch
[Picked up by 282 media titles]
2026-03-05 17:14