Medical/Pharmaceuticals
Qilian International Holding Group Limited Releases 2022 Chairman Letter
JIUQUAN, China, Jan. 11, 2022 /PRNewswire/ -- Qilian International Holding Group Limited (Nasdaq: QLI) (the "Company", "Qilian International", "we", "our" or "us"), aChina-based pharmaceutical and chemical products manufacturer, today released a letter to shareholders from the Chairman of the Com...
China's 2021 U.S. Patent Grants Surged, Even as Total Patent Awards Trended Downward, According to Analysis by IFI CLAIMS
2021 Rankings Shed Light on Corporate R&D Priorities: Telemedicine, Supply Chain Efficiencies, E-Cigs and Artificial Intelligence Among Hottest Areas of Activity NEW HAVEN, Conn., Jan. 11, 2022 /PRNewswire/ -- Enhancements in telemedicine, more hardy grains to feed the world in a time of climate...
Fosun Pharma and Insilico Medicine Announce a Strategic, AI-driven Drug Discovery and Development Collaboration to Jointly Advance Multiple Targets
SHANGHAI and NEW YORK, Jan. 11, 2022 /PRNewswire/ -- Shanghai Fosun Pharmaceutical (Group) Co., Ltd ("Fosun Pharma"; Stock Code: 600196.SH, 02196.HK), a leading innovation-driven international healthcare group inChina and Insilico Medicine ("Insilico"), an end-to-end artificial intelligence (AI)...
Latest Study Shows Encouraging Results of CanSinoBIO's Inhaled COVID-19 Vaccine as Heterologous Booster
TIANJIN, China, Jan. 11, 2022 /PRNewswire/ -- CanSino Biologics Inc. ("CanSinoBIO") (SSE: 688185, HKEX: 06185) today announced thatPreprints with The Lancet, a collaboration between the research sharing platform SSRN and The Lancet, published a clinical study on the safety and immunogenicity of ...
SNIPR BIOME Announces FDA Clearance of Investigational New Drug (IND) Application for SNIPR001, a Novel CRISPR Therapy Targeting Life-Threatening E. coli Infections
COPENHAGEN, Denmark, Jan. 11, 2022 /PRNewswire/ -- SNIPR BIOME ApS, a leading CRISPR and microbiome biotechnology company, is pleased to announce that the US Food and Drug Administration (FDA) has approved the Investigational New Drug (IND) Application, for our first development candidate, enabli...
MGI Aims to Boost its Market Share through Participation at Expo 2020 Dubai's Guangdong Week
DUBAI, UAE, Jan. 11, 2022 /PRNewswire/ -- Global life science innovator MGI Tech Co. Ltd. (MGI) is aiming to boost its market share in theMiddle East and Asia through participation at Guangdong Week, a dedicated event taking place at the world exhibition's China Pavilion fromJanuary 11-13. The ...
Pfizer Drives Cultural Change in Sustainable and Accessible Data with MicroStrategy
SINGAPORE, Jan. 11, 2022 /PRNewswire/ -- MicroStrategy
Keep Your Home Safe and Build a Protected Community Against Omicron with Circle HealthPod
* Circle HealthPod detects >99.9% of known COVID-19 strains, including the Omicron variant, in approximately 20 minutes * With technology developed at the University of Oxford, the Circle HealthPod is a CE-IVD marked rapid detection system with 98.4% molecular accuracy * Frequent testing wit...
Ascletis Announces First Patient Dosed in the Phase II Clinical Trial of ASC42, An In-House Developed, Best-in-Class FXR Agonist for Chronic Hepatitis B Indication
HANGZHOU, China and SHAOXING, China, Jan. 11, 2022 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX: 1672) today announces the dosing of the first patient in the Phase II clinical trial of ASC42 for chronic hepatitis B (CHB) indication. The Phase II clinical trial (ClinicalTrials.gov Identifier: NCT051...
Dr. Ivan Puah Highlights The Significant Impact Of Covid That Changes Our Priority In Life
SINGAPORE, Jan. 11, 2022 /PRNewswire/ -- The current pandemic has raised more awareness of the importance of health and better care of oneself. Dr.Ivan Puah, Medical Director at Amaris B. Clinic shares how people's lifestyles and priorities have changed since Covid-19 started. 01. Losing we...
Sana Biotechnology, IASO Biotherapeutics, and Innovent Biologics Announce Non-Exclusive License Agreement for Clinically Validated BCMA CAR Construct
SEATTLE, SAN FRANCISCO, SAN JOSE, Calif., NANJING, China, SUZHOU, China and SHANGHAI, Jan. 11, 2022 /PRNewswire/ -- Sana Biotechnology, Inc. (NASDAQ: SANA), a company focused on creating and delivering engineered cells as medicines, IASO Biotherapeutics ("IASO Bio"), a clinical-stage biopharmace...
Sana Biotechnology, IASO Biotherapeutics, and Innovent Biologics Announce Non-Exclusive License Agreement for Clinically Validated BCMA CAR Construct
SEATTLE and SAN FRANCISCO and SAN JOSE, Calif. and NANJING, China and SUZHOU, China andSHANGHAI, Jan. 10, 2022 /PRNewswire/ -- Sana Biotechnology, Inc. (NASDAQ: SANA), a company focused on creating and delivering engineered cells as medicines, IASO Biotherapeutics ("IASO Bio"), a clinical-stage ...
Trajan Group acquires Neoteryx LLC
MELBOURNE, Australia, Jan. 10, 2022 /PRNewswire/ -- Global analytical science and device company Trajan Group Holdings Limited (ASX: TRJ) (Trajan or the Company) has completed the acquisition of Neoteryx, LLC (Neoteryx), a global leader in blood microsampling devices based inTorrance, California,...
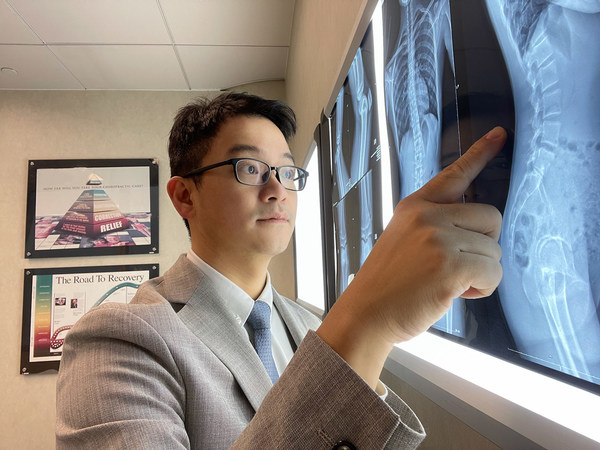
The new symptoms of the COVID period that CDAHK doctors have uncovered
HONG KONG, Jan. 10, 2022 /PRNewswire/ -- As the COVID-19 Omicron variety continues to spread, chiropractors see a spike in neck pathology from work-from-home, remote learning during pandemic. For 30 years before the COVID-19 pandemic, the majority of chiropractic patients were suffering from bac...
CARsgen Announces CT041 CAR T-cell Product Candidate Granted RMAT Designation by the FDA
SHANGHAI, Jan. 10, 2022 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company mainly focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, today announced thatthe United States Food and Drug Administration (FDA)...
New Resource from NCCN Shares Evidence-Based Approaches to Recognize and Manage Graft-Versus-Host Disease after Stem Cell Transplantation
PLYMOUTH MEETING, Pa., Jan. 10, 2022 /PRNewswire/ -- The National Comprehensive Cancer Network (NCCN®) today announced the publication of new NCCN Guidelines for Patients®: Graft-Versus-Host Disease (GVHD). GVHD is a complication that occurs after a donor stem cell or bone marrow (a.k.a. hematopo...
Everest Medicines Will Participate in a Clinical Trial with Gilead and MSD to Evaluate Trodelvy® in Combination with KEYTRUDA® (pembrolizumab) in First-Line Metastatic Non-Small Cell Lung Cancer
SHANGHAI, Jan. 10, 2022 /PRNewswire/ -- Everest Medicines
Clinical study on Bactiguard's endotracheal tube now published - shows significant reduction of ventilator-associated pneumonia
STOCKHOLM, Jan. 10, 2022 /PRNewswire/ -- The VITAL study, by Professor Pierre Damas and his team, is now published in the well renowned journal Annals of Intensive Care. The study shows a 53% reduction of ventilator-associated pneumonia with Bactiguard's endotracheal tube. It was presented for th...
Clarivate Identifies Seven Potential Blockbuster Drugs in Annual Drugs to Watch Report
Despite the challenges of pandemic disruptions, drug developers advance promising therapies for conditions, including Alzheimer's, diabetes and asthma Report also examines key therapeutic development areas to watch including mRNA, CRISPR, AI-driven drug discovery and more LONDON, Jan. 10, 2022 /...
Ascletis Announces U.S. IND Filing for In-House Developed Oral PD-L1 Small Molecule Inhibitor ASC61 for Treatment of Advanced Solid Tumors
HANGZHOU, China and SHAOXING, China, Jan. 10, 2022 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX: 1672) announces today the filing of the U.S. Investigational New Drug (IND) application for in-house developed oral PD-L1 small molecule inhibitor, ASC61, for the treatment of advanced solid tumors. AS...
Week's Top Stories
Most Reposted
LINKDOOD Breaks Language Barriers, Ushering a New Era for Cross-Border Romance
[Picked up by 326 media titles]
2024-04-29 06:00Dow showcases circular and innovative materials science solutions and industry collaborations at Chinaplas 2024
[Picked up by 291 media titles]
2024-04-30 10:11Puyuan Fashion Resort 2024: A Grand Unveiling of Global Trends and Local Heritage
[Picked up by 269 media titles]
2024-04-28 09:39Chinese new energy industry contributes to global green, low-carbon transition
[Picked up by 249 media titles]
2024-04-30 15:13New International Land-Sea Trade Corridor achieves rapid development
[Picked up by 246 media titles]
2024-04-28 10:24