Medical/Pharmaceuticals
Yushangmei seeks to establish partnership with a new target company
GUANGZHOU, China, July 8, 2021 /PRNewswire/ -- Guangzhou Yushangmei Health Management Co., Ltd. announced the termination of the partnership with the company headquartered inNevada, USA. Meanwhile, Yushangmei has sought to establish partnership with a new target company, specific details will be ...
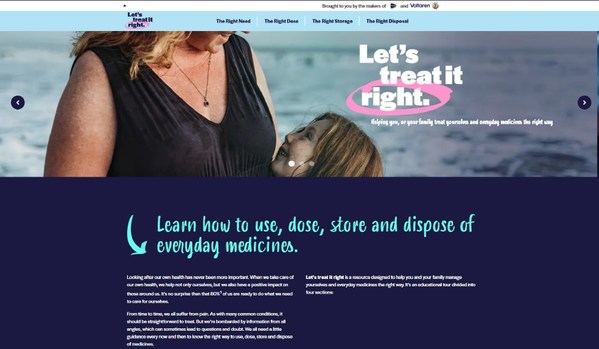
COVID pandemic heightens a lack of confidence amongst the public about which medicines to take and how to use them
New campaign, Let's treat it right, launches as online searches for self-care surge globally[1], as people take more responsibility for their everyday health. NYON, Switzerland, July 8, 2021 /PRNewswire/ -- Today sees the launch of Let's treat it right, a major new health education campaign d...
Nippon Express (India) Newly Acquires GDP Certification at Three Locations
TOKYO, July 8, 2021 /PRNewswire/ -- Nippon Express (India) Private Limited (hereinafter "NE India"), a local subsidiary of Nippon Express Co., Ltd., obtained Good Distribution Practice (GDP) certification in April for its temperature-controlled hubs inDelhi, Mumbai and Ahmedabad, evidencing the ...
COVID-19: Doctor's certificates for antigen self-tests now offered online worldwide by DrAnsay.com
HAMBURG, Germany, July 8, 2021 /PRNewswire/ -- DrAnsay.com, the market leader
for online doctor attests, now offers online test certificates for self-applied
rapid antigen tests worldwide to avoid the effort, infection risk and cost of
point-of-care testing.
BioShin Expands Senior Leadership Team: Sarah Lu Joins BioShin as Chief Medical Officer, Mary Ma Joins BioShin as Chief Commercial Officer
SHANGHAI, July 8, 2021 /PRNewswire/ -- BioShin Limited today announced the appointment of Dr.Zhihong (Sarah) Lu as Chief Medical Officer and Dr. Ma (Mary) Li as Chief Commercial Officer. Sarah will play a critical role in BioShin's clinical development of pipeline products inAsia-Pacific markets,...
Just 5 minutes: Game-changing PCR to complete nucleic acid amplification, Si-Gene Biotech help out against COVID 19
SHANGHAI, July 8, 2021 /PRNewswire/ -- As is known, nucleic acid detection is currently the "golden standard" of coronavirus detection and has the high sensitivity and specificity characteristics in early diagnosis. The most common method for detecting novel coronavirus-specific nucleic acid sequ...
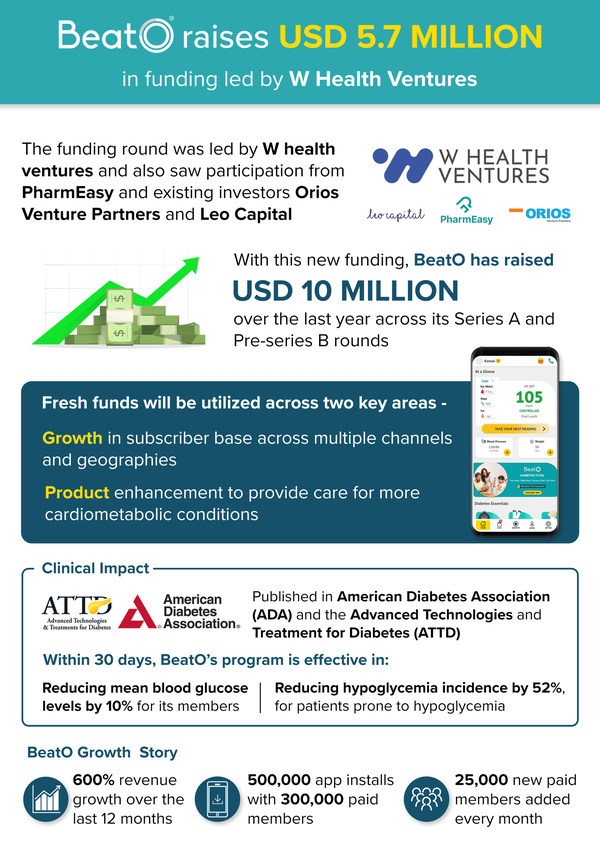
BeatO raises USD 5.7 million in funding led by W Health Ventures
- Fresh funds to be utilized in enlarging its subscriber base across channels/geographies and for product enhancement to provide care for more cardiometabolic conditions NEW DELHI, July 8, 2021 /PRNewswire/ -- BeatO – India's leading digital care ecosystem for chronic condition management – has ...
CStone announces updated results for AYVAKIT(R) (avapritinib) in Chinese patients with advanced GIST at ESMO World Congress on Gastrointestinal Cancer 2021
* AYVAKIT has demonstrated significant anti-tumor activity in Chinese patients with PDGFRA D842V mutant gastrointestinal stromal tumor (GIST), and has also shown potential for the treatment of fourth-line GIST * AYVAKIT had a generally well-tolerated safety profile in Chinese patients with GI...
Cambridge Isotope Labs Announces the First Commercially Available Quantum-Grade Gas, 12C Methane QG-Alpha™ for High-Performance Nitrogen-Vacancy Center Diamonds
TEWKSBURY, Mass., July 8, 2021 /PRNewswire/ -- Cambridge Isotope Laboratories, Inc. (CIL), part of the Otsuka Group and the world's leading producer of stable isotopes and stable isotope-labeled compounds, is proud to launch the first commercially available 12C-enriched methane-grade gas for quan...
Otsuka Signs Three-Year Collaboration with Holmusk to Enhance Digital Health and Data Analytics for Global Behavioral Health Programs
PRINCETON, N.J., July 8, 2021 /PRNewswire/ -- Otsuka Pharmaceutical Development & Commercialization, Inc. ("Otsuka"), announces today that it has entered a three-year collaboration with Holmusk Inc. ("Holmusk"), a global data science and digital health company building the world's largest real-wo...
Ortho Clinical Diagnostics Announces Partnership With Thermo Fisher Scientific
Introducing VITROS® QC Solutions, taking your lab to the next level with Thermo Scientific™ MAS™ Quality Controls RARITAN, N.J., July 7, 2021 /PRNewswire/ -- Ortho Clinical Diagnostics (Nasdaq: OCDX), one of the world's largest pure-play, in-vitro diagnostics (IVD) companies, today announced its...
dMed-Clinipace Announces Completion of US$50 Million Series C+ Financing from New Investors
SHANGHAI and MORRISVILLE, N.C., July 7, 2021 /PRNewswire/ -- dMed-Clinipace, the global full-service Clinical Contract Research Organization (CRO), announced today the recent successful completion of aUS$50 million Series C+ financing with participation from a group of new, leading international ...
Biocytogen/Eucure Biopharma's CTLA-4 and CD40 mAbs Approved for Phase II Clinical Trials by the FDA
BOSTON and BEIJING, July 7, 2021 /PRNewswire/ -- Eucure Biopharma, a wholly
owned subsidiary of Biocytogen
Elekta optimizes treatment times for cancer patients via proactive service innovation
STOCKHOLM, July 7, 2021 /PRNewswire/ -- For the last several years, Elekta (EKTA-B.ST) has invested substantially in digitized service solutions to keep critical treatment machines up and running at cancer clinics, thereby ensuring that patients receive timely radiotherapy. Among these solutions ...
Innophys' MUSCLE SUIT Selling Steadily after Topping 20,000 Units
TOKYO, July 7, 2021 /PRNewswire/ -- Developed and sold by Innophys Co., Ltd., MUSCLE SUIT has been selling steadily even after exceeding 20,000 units in cumulative total (*1) all over the world recently, making it a fast-selling (*2) exoskeleton assist suit in the world. Notes: (*1) Total numbe...
CStone announces China NMPA acceptance of IND application for CS2006/NM21-1480, a PD-L1/4-1BB/HSA multi-specific antibody-based molecule, marking further expansion of its Pipeline 2.0 strategy
SUZHOU, China, July 7, 2021 /PRNewswire/ -- CStone Pharmaceuticals ("CStone", HKEX: 2616), a leading biopharmaceutical company focused on the research, development, and commercialization of innovative immuno-oncology therapies and precision medicines, today announced that the investigational new ...
New Cancer Treatment For Australian Dogs With APVMA Approval Of Qbiotics' Stelfonta®
* STELFONTA® (tigilanol tiglate) is now approved by the Australian Pesticides and Veterinary Medicines Authority (APVMA) for the treatment of canine non-metastatic mast cell tumours (MCT). * APVMA approval follows marketing authorisation (registration) and launch of STELFONTA® in the major co...
Immunogenicity Expert and FDA Alum Dr. Amy Rosenberg Joins EpiVax
PROVIDENCE, R.I., July 7, 2021 /PRNewswire/ -- EpiVax, Inc. ("EpiVax") is pleased to announce that Dr.Amy Rosenberg is leaving the FDA's Division of Therapeutic Proteins, CDER, which she led for over 20 years, to join EpiVax as Senior Director of Immunology and Protein Therapeutics. Dr. Rosenber...
Transcenta Announces NMPA Acceptance of IND Application of its Anti-Sclerostin Monoclonal Antibody TST002
SUZHOU, China, July 6, 2021 /PRNewswire/ -- Transcenta Holding Limited ("Transcenta"), a clinical stage global biotherapeutics company with fully-integrated capabilities in discovery, development and manufacturing of antibody-based therapeutics, today announces that NMPA has accepted IND applica...
Harbour BioMed Reports Positive Topline Results from Phase 2 Trial of Batoclimab (HBM9161) in Generalized Myasthenia Gravis
* Both batoclimab 340 mg and 680 mg treatment groups demonstrated rapid, substantial and persistent clinical improvement over placebo * Favorable safety and tolerability profile with no serious adverse events (SAEs) and no adverse events (AEs) leading to discontinuation * Strong IgG reductio...
Week's Top Stories
Most Reposted
Troy Animal Healthcare Strengthens Quality Operations with Veeva Vault Quality
[Picked up by 328 media titles]
2024-05-17 07:00Ultima Markets Recognised as Most Popular Broker 2024 by BrokersView
[Picked up by 321 media titles]
2024-05-15 12:00Innomix and World Castle Association Pioneer LBMR project at Zhangbi Fortress
[Picked up by 320 media titles]
2024-05-20 13:48Samsung Electronics Unveils Month-Long Celebration of Esports Events for Students in Southeast Asia
[Picked up by 317 media titles]
2024-05-21 08:00Home to half of the world's top 10 trending tourism destinations, Asia Pacific is making a comeback: Mastercard Economics Institute on travel in 2024
[Picked up by 308 media titles]
2024-05-16 15:00