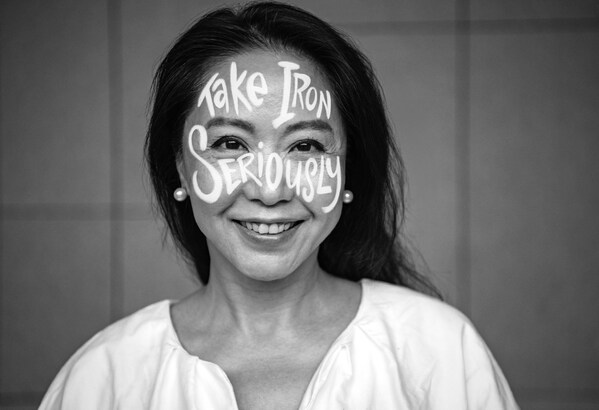Pharmaceuticals
Triple Negative Breast Cancer: Expert Consensus on Local Treatment Options
Hong Kong Breast Cancer Foundation Launches "Immunotherapy Drug Financial Assistance Programme" to Benefit Patients in Need HONG KONG, Nov. 30, 2023 /PRNewswire/ -- Breast cancer is the leading cancer facing women inHong Kong. According to the statistics released by the Hong Kong Cancer Registry...
111, Inc. Announces Third Quarter 2023 Unaudited Financial Results
SHANGHAI, Nov. 30, 2023 /PRNewswire/ -- 111, Inc. ("111" or the "Company") (NASDAQ: YI), a leading tech-enabled healthcare platform company committed to digitally connecting patients with medicine and healthcare services inChina, today announced its unaudited financial results for the third quart...
Everest Medicines Announces Acceptance of Nefecon® New Drug Applications for the Treatment of Primary IgA Nephropathy in Adult Patients in South Korea
SHANGHAI, Nov. 30, 2023 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK, "Everest", or the "Company"), a biopharmaceutical company focused on the development, manufacturing and commercialization of innovative medicines and vaccines, announced today that its New Drug Application (NDA) for Nefecon®...
Phase II OPALESCENCE Study of TLX250-CDx in Triple Negative Breast Cancer to be Presented at SABCS
MELBOURNE, Australia, Nov. 30, 2023 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Telix, the Company) today advises that the completed Phase II OPALESCENCE investigator-initiated trial (IIT) of its carbonic anhydrase IX (CAIX)-targeting positron emission tomography (PET) imaging candid...
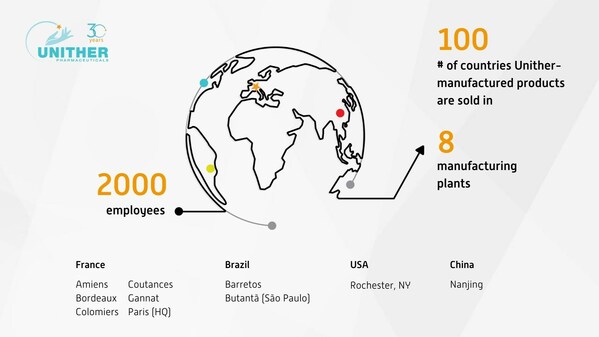
Unither Pharmaceuticals celebrates 30 years of innovation and expertise
PARIS, Nov. 30, 2023 /PRNewswire/ -- Unither Pharmaceuticals, a leading CDMO (Contract Development and Manufacturing Organization), celebrates 30 years in business. In 1993, Unither Pharmaceuticals began operations by acquiring a 17-man pharmaceutical plant that produced 3 pharmaceutical forms. T...
Clarity's theranostic prostate cancer trial progresses at the highest dose level cohort
HIGHLIGHTS * Cohort 3 of the theranostic SECuRE trial investigating 64Cu/67 Cu-SAR-bisPSMA in metastatic castrate-resistant prostate cancer (mCRPC) has enrolled and treated 3 participants who received therapy with67Cu-SAR-bisPSMA at the highest dose level of 12GBq. * No dose limiting toxicit...
China Pharma Holdings, Inc. Announced the Completion of Third Party Testing of Dry Eye Disease Therapeutic Device
HAIKOU, China, Nov. 29, 2023 /PRNewswire/ -- China Pharma Holdings, Inc. (NYSE American: CPHI) ("China Pharma", or the "Company"), an NYSE American-listed corporation with a fully-integrated specialty pharmaceuticals subsidiary based inChina, today announced that its Dry Eye Disease (DED) therape...
VISTA Eye Specialist Unveils Innovative Solution for Reading Problems
PETALING JAYA, Malaysia, Nov. 29, 2023 /PRNewswire/ -- VISTA Eye Specialist (VISTA) proudly introduces the groundbreaking EVO Viva Implantable Collamer Lens (Viva) to address the dual challenge of short-sightedness and reading problems (presbyopia) among patients aged above 40. This cutting-edge ...
Dr. Liu Jian, President of Medicilon Drug Discovery Division, wins the Thomas Alva Edison Patent Award
BOSTON, Nov. 28, 2023 /PRNewswire/ -- Dr. Liu Jian, President of the Drug Discovery Division at Medicilon, has been awarded the Thomas Alva Edison Patent Award for 2023 from the New Jersey Research and Development Committee. This recognition stems from his innovative patent on the PCSK9 inhibitor...
Akeso Launches Construction of Shanghai Global R&D Center to Accelerate Its Innovation Globalization Strategy
HONG KONG, Nov. 28, 2023 /PRNewswire/ -- The groundbreaking ceremony for Akeso (9926.HK) Shanghai Global R&D Center was successfully held at the Zhangjiang headquarters park inShanghai. The establishment of the Akeso Global R&D Center signifies a crucial step in Akeso's innovative globalization ...
Novavax's Updated Protein-based COVID-19 Vaccine Now an Option for All 194 Member States of the World Health Organization
GAITHERSBURG, Md., Nov. 28, 2023 /PRNewswire/ -- Novavax, Inc. (Nasdaq: NVAX), a global company advancing protein-based vaccines with its Matrix-M™ adjuvant, today announced that Nuvaxovid™ XBB.1.5 COVID-19 Vaccine (NVX-CoV2601) has been granted Emergency Use Listing (EUL) by the World Health Or...
RemeGen Announces Publication of Results from Two Phase II Studies for Disitamab Vedotin in Latest Issue of Journal of Clinical Oncology (JCO)
YANTAI, China, Nov. 28, 2023 /PRNewswire/ -- RemeGen Co. Ltd.
Daewoong Pharmaceutical Received GMP Certification from Brazilian ANVISA
SEOUL, South Korea, Nov. 28, 2023 /PRNewswire/ -- Daewoong Pharmaceutical is pleased to announce that its Osong factory in Korea achieved Good Manufacturing Practice (GMP) certification from the Regulatory Authority ofBrazil, ANVISA. Daewoong Pharmaceutical, represented by CEO Seng-ho Jeon and ...
Cadonilimab (PD-1/CTLA-4 Bispecific Antibody) Met Primary Endpoint of Progression-Free Survival in Phase III Trial for First-Line Treatment of Recurrent/Metastatic Cervical Cancer in All-Comer Patients
HONG KONG, Nov. 27, 2023 /PRNewswire/ -- Akeso (9926. HK) has announced that the AK104-303 Phase III trial, which investigated cadonilimab (Akeso's PD-1/CTLA-4 bispecific antibody) combined with platinum-based chemotherapy +/- bevacizumab, has met one of its primary endpoints of progression-fre...
DxVx, plans to make a license-in agreement of OVM-200 and conduct clinical trials in Asia
- Final stage in licensing OVM-200 from Oxford Vacmedix in the UK - DxVx plans to proceed its own clinical trials in Asia, including Korea and China SEOUL, South Korea, Nov. 24, 2023 /PRNewswire/ -- DxVx announces today that it will conduct its own clinical trials of OVM-200, an anti-cancer vacc...
Ascentage Pharma Hosts Ceremony Marking the Launch of Olverembatinib in Newly Approved Indication and the Dispatch of First Batch for the New Indication
SUZHOU, China, and ROCKVILLE, Md., Nov. 24, 2023 /PRNewswire/ -- 23 Nov, 2023, on the second anniversary of the initial approval for olverembatinib, Ascentage Pharma hosted a ceremony in Suzhou,China, marking the launch of olverembatinib in a newly approved indication and the dispatch of the firs...
China's First NDA for a KRAS G12C Inhibitor: NMPA Accepts New Drug Application for GFH925 and Grants GFH925 with Priority Review Designation
SHANGHAI, Nov. 24, 2023 /PRNewswire/ -- GenFleet Therapeutics, a clinical-stage biotechnology company focusing on cutting-edge therapies in oncology and immunology, today announced the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) ofChina has accepted the...
Iron Deficiency Day 2023: CSL Vifor calls for access to iron deficiency diagnosis and treatment
Iron Deficiency Day unites a strong global coalition raising awareness on the serious health impact of iron deficiency and iron deficiency anemia1 This year, the spotlight is on the importance of early iron deficiency diagnosis and treatment and its impact on quality of life ST. GALLEN, SWITZERL...
Everest Medicines Announces China NMPA's Approval of Nefecon® for the Treatment of Primary IgA Nephropathy in Adult Patients
--Nefecon® is the first approved medicine with an IgAN indication in China-- --The approval marks a new era of IgAN treatment for Chinese patients-- SHANGHAI, Nov. 24, 2023 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK, "Everest", or the "Company"), a biopharmaceutical company focused on the ...
China's First NDA for a KRAS G12C Inhibitor: Innovent Announces the National Medical Products Administration of China Has Accepted and Granted Priority Review Designation to the New Drug Application for IBI351
ROCKVILLE, Md. and SUZHOU, China, Nov. 24, 2023 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, metabolic, autoimmune, ophthalmology an...
Week's Top Stories
Most Reposted
QDX Co-Founder Giuseppe Barca Awarded Prestigious Gordon Bell Prize, Pioneering Advancements in Drug Discovery Technology
[Picked up by 303 media titles]
2024-11-26 18:49ITAP 2024 sets the stage for a more connected advanced factory ecosystem with focus on AI, advanced robotics and sustainability
[Picked up by 296 media titles]
2024-11-28 17:24Southeast Asian Consumers Raise Online Security Concerns, New GSMA Survey Shows
[Picked up by 296 media titles]
2024-11-26 13:00MediSun Energy Raises $8.75M Seed Round with Vynn Capital to Drive MENA Expansion and Advance Osmotic Energy Innovation
[Picked up by 280 media titles]
2024-11-25 10:00Announcement of "Cyber Sousa Award" Winners of the 16th Xiamen International Animation Festival
[Picked up by 272 media titles]
2024-11-26 16:48