Pharmaceuticals
TraceLink's Magnum Release of its Opus Platform Will Revolutionize Supply Chain Management by Enabling All Businesses to Digitalize their End-to-End Supply Networks
With real-time information from all supply chain partners, Supply Chain Managers can reduce inventory and stock-out while improving service levels and lead times BOSTON, Sept. 4, 2024 /PRNewswire/ -- TraceLink, the largest end-to-end digital network platform for intelligent orchestration of the ...
LakeShore Biopharma Announces Leadership Transitions
GAITHERSBURG, Md., Sept. 4, 2024 /PRNewswire/ -- LakeShore Biopharma Co., Ltd. (Nasdaq: LSB) ("LakeShore Biopharma" or the "Company"), a global biopharmaceutical company dedicated to discovering, developing, manufacturing, and delivering new generations of vaccines and therapeutic biologics for ...
LakeShore Biopharma Announces Leadership Transitions
GAITHERSBURG, Md., Sept. 4, 2024 /PRNewswire/ -- LakeShore Biopharma Co., Ltd. (Nasdaq: LSB) ("LakeShore Biopharma" or the "Company"), a global biopharmaceutical company dedicated to discovering, developing, manufacturing, and delivering new generations of vaccines and therapeutic biologics for ...
HKBU develops Chinese medicine for ulcerative colitis approved by National Medical Products Administration for clinical trial
HONG KONG, Sept. 4, 2024 /PRNewswire/ -- The Centre for Chinese Herbal Medicine Drug Development (CDD) at Hong KongBaptist University (HKBU) has achieved a significant milestone in developing a novel Chinese herbal formulation for ulcerative colitis remission maintenance. Following a submission o...
Innovent Receives Fast Track Designation from the U.S. FDA for IBI363 (PD-1/IL-2α Bispecific Antibody Fusion Protein) as Monotherapy for Advanced Melanoma
SAN FRANCISCO and SUZHOU, China, Sept. 4, 2024 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, cardiovascular and metabolic, autoimmune...
IPAX-1 Study of TLX101 Investigational Glioblastoma Therapy Published in Neuro-Oncology Advances
MELBOURNE, Australia, Sept. 4, 2024 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Telix, the Company) today announces that the Company's IPAX-1 Phase I study has been published inNeuro-Oncology Advances, confirming the safety and tolerability profile, and early efficacy of TLX101 thera...
Biostar Announces Completion of Patient Recruitment for US Phase 1 Clinical Study of Utidelone Capsule
SAN FRANCISCO, Sept. 3, 2024 /PRNewswire/ -- Biostar Pharma, Inc., the US subsidiary of Beijing Biostar Pharmaceuticals Co., Ltd. which is a synthetic biology driven biopharma company focusing on the discovery, development and commercialization of innovative oncology drugs, is pleased to announce...
Skyline Therapeutics' Novel Gene Therapy SKG1108 Receives FDA Orphan Drug Designation for Retinitis Pigmentosa
BOSTON and SHANGHAI, Sept. 3, 2024 /PRNewswire/ -- Skyline Therapeutics, an innovation-driven gene therapy company committed to developing unique and novel solutions for rare and severe diseases, announced that the US Food and Drug Administration (FDA) has granted Orphan Drug Designation (ODD) fo...
GC Biopharma and Hanmi Pharmaceutical Receives IND Clearance for Phase 1/2 Clinical Trial from the U.S. FDA
* Co-development of innovative new drug for the treatment of Fabry disease as "the world's first once-monthly subcutaneous treatment" * Improves efficacy compared to existing treatment for kidney function, vascular disease, and peripheral nerve disorders YONGIN, South Korea, Sept. 3, 2024 /PR...
Caliway Announces the Initiation of Subject Recruitment in CBL-514 Phase 2b Study for Dercum's Disease
NEW TAIPEI CITY, Sept. 3, 2024 /PRNewswire/ -- Caliway Biopharmaceuticals (Caliway) announced that the subject recruitment of CBL-514 Phase2b study for Dercum's disease (CBL-0202 DD Phase2b study, NCT06303570) has been initiated. The study results are anticipated in Q4 2025. CBL-0202DD study i...
Seegene and Springer Nature Launch 'Nature Awards MDx Impact Grants' to Innovate Syndromic PCR Diagnostic Assays
- Nature Awards MDx Impact Grants in partnership with Seegene extends commitment to external innovation. - Global research teams invited to submit proposals to develop new tests for human infectious diseases. Application deadline:December 2, 2024. - Nature Awards to lead the application and eva...
Simcere Zaiming collaborates with TargetRx to introduce a third-generation ALK inhibitor
NANJING, China, Sept. 2, 2024 /PRNewswire/ -- On September 02, 2024, Simcere Zaiming, an innovative oncology company under Simcere Pharmaceutical Group (2096.HK), announced a collaboration agreement with Shenzhen TargetRx Inc. The partnership focuses on the ALK/ROS1 dual receptor tyrosine kinase ...
Frost & Sullivan Released Report: "BiBo Pharma: A Global CDMO Leading in Advanced Biopharmaceutical Manufacturing Technologies"
SHANGHAI, Sept. 2, 2024 /PRNewswire/ -- Frost & Sullivan released a report, titled "BiBo Pharma: A Global CDMO Leading in Advanced Biopharmaceutical Manufacturing Technologies". The report explores a global CDMO leading the "The Fourth Revolutionary Wave of Biologics Manufacturing" and pioneering...
Innovent Annouces Multiple Clinical Study Results of Mazdutide to be Presented at the EASD 2024
SAN FRANCISCO and SUZHOU, China, Sept. 2, 2024 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, autoimmune, cardiovascular and metabolic...
Pierre Fabre Laboratories receives European Commission Approval for BRAFTOVI®(encorafenib) in combination with MEKTOVI® (binimetinib) for the treatment of adult patients with advanced non-small cell lung cancer (NSCLC) with a BRAFV600E mutation
* European approval is based on results from the Phase II PHAROS trial, which showed a meaningful clinical benefit to BRAFV600E mutated advanced NSCLC patients with an objective response rate (ORR) of 75% in treatment-naïve patients and 46% in previously treated patients.[1-3] The safety profil...
Jacobio Out-licensed KRAS G12C Inhibitor Glecirasib and SHP2 Inhibitor JAB-3312 to Allist in China
BEIJING and SHANGHAI and BOSTON, Aug. 30, 2024 /PRNewswire/ -- Jacobio Pharma (1167.HK), a clinical-stage oncology company focusing on undruggable targets, today announced that it has granted theChina rights (including mainland China, Hong Kong, Macau, and Taiwan) of KRAS G12C inhibitor glecirasi...
Breaking News | Sanyou Bio Launches Comprehensive Monkeypox Product Line
SHANGHAI, Aug. 30, 2024 /PRNewswire/ -- On August 28, 2024, Sanyou Biopharmaceuticals (Shanghai) Co., Ltd. announced the launch of a comprehensive product line targeting monkeypox, which includes antigens, monoclonal antibodies, and overexpression cell lines. This product line features 65 items ...
Guardant Health Japan receives regulatory approval of Guardant360® CDx liquid biopsy as companion diagnostic for amivantamab-vmjw to identify patients with inoperable or recurrent NSCLC harbouring EGFR exon 20 insertion mutations
* Guardant360® CDx is the first blood-based companion diagnostic to be approved inJapan for the detection of EGFR exon 20 insertion mutations TOKYO, Aug. 30, 2024 /PRNewswire/ -- Guardant Health Japan Corp. (HQ: Minato-ku, Tokyo / Representative Director:Mika Takaki), a leading precision oncolog...
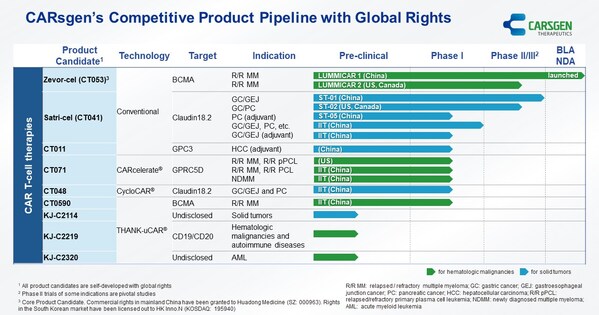
CARsgen® Announces 2024 Interim Results
SHANGHAI, Aug. 29, 2024 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, has announced its 2024 Interim Results. Business Highlights * Zevor-cel was...
Viva Biotech Announces Its 2024 Interim Results: Significant Improvement in Net Profit Growth and Ongoing Optimization of Emerging Technology Platforms
Results Highlights for Interim Results ended 30 June 2024 Revenue reached RMB981.8 million Gross profit amounted to RMB339.1 million Net profit grew by 956.0 YoY to RMB144.2 million Adjusted non-IFRS net profit reached RMB168.2 million, increased by 15.1% YoY HONG KONG, Aug. 29, 2024 /PRNewswire/ ...
Week's Top Stories
Most Reposted
Agoda Launches Agoda Impact Lab at ASEAN Tourism Forum
[Picked up by 322 media titles]
2026-01-29 15:06Wonder Raises USD 12 Million Venture Debt from HSBC Innovation Banking to Drive Growth and Expansion
[Picked up by 322 media titles]
2026-02-02 10:00AI adoption is widespread, but developer confidence is still catching up, Agoda report finds
[Picked up by 312 media titles]
2026-02-03 11:00Mastercard Launches Portfolio of Fleet Solutions in Asia Pacific
[Picked up by 308 media titles]
2026-02-04 09:00Colebrook Bosson Saunders Officially Launches Lana, A Circular Ergonomic Laptop Stand for the Hybrid Generation
[Picked up by 303 media titles]
2026-02-03 12:00