Pharmaceuticals
ESMO 2023 | Ascentage Pharma to Present Results from Two Clinical Studies, including One Oral Presentation, at 2023 ESMO Congress
SUZHOU, China, and ROCKVILLE, Md., Aug. 2, 2023 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancer, chronic hepatitis B (CHB), and age-related diseases, announced today that it is going to release results from two clinic...
SK Biopharmaceuticals Assembles 'Board of Prominent Experts' to Boost Growth, Innovation
Renowned oncologist Yung-Jue Bang, M.D., Ph.D., chairs the distinguished 5-member Scientific Advisory Board to further advance SK Biopharmaceuticals as a global big biotech SEOUL, South Korea, Aug. 2, 2023 /PRNewswire/ -- SK Biopharmaceuticals, a global biotech company, announced the formation o...
Arbele announces early safety data for novel CDH17xCD3 bispecific T-cell engager at American Society of Clinical Oncology Breakthrough Meeting
SYDNEY, Aug. 2, 2023 /PRNewswire/ -- Arbele, a clinical-stage biotechnology company developing novel immunotherapies targeted for advanced gastrointestinal cancers, presented early safety data from its first-in-class CDH17xCD3 bispecific T-cell engager antibody, ARB202, at the ASCO Breakthrough c...
Unlocking the Power of Genetics: Camtech Unveils DNA Self-Test Kits for Holistic Wellness
SINGAPORE, Aug. 2, 2023 /PRNewswire/ -- Camtech Diagnostics, a provider of
innovative healthcare testing solutions, is excited to announce the launch of
its Ideal Health DNA Test
Selected for oral presentation! Kelun-Biotech to announce latest clinical study results of SKB264 (MK-2870, TROP2-ADC) for treatment of HR+/HER2- breast cancer at ESMO Congress 2023
CHENGDU, China, Aug. 1, 2023 /PRNewswire/ -- Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. ("Kelun-Biotech", HKEX: 6990.HK) announced that it will present the data from a Phase I/II clinical trial of the innovative TROP2-ADC (SKB264, MK-2870) jointly developed by Kelun-Biotech and MSD (the t...
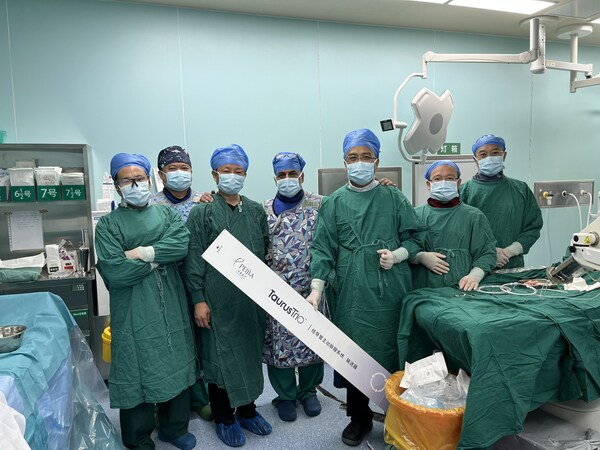
Peijia Medical Announces First Chinese Patient Implanted with TaurusTrio™ Transcatheter Aortic Valve Replacement System
SUZHOU, China, Aug. 1, 2023 /PRNewswire/ -- Peijia Medical (HKG:9996), a leading Chinese domestic player in the high-growth transcatheter valve therapeutic and neurointerventional procedural medical device markets, announced onJuly 31, 2023 that the first Chinese patient of the multi-center ...
Doer Biologics Announces First Subject Dosed in Phase I 12-Week Multiple-Ascending Dose (MAD) Clinical Trial of DR10624 and Received IND Approval From NMPA
HANGZHOU, China, Aug. 1, 2023 /PRNewswire/ -- Zhejiang Doer Biologics Co., Ltd. ("Doer Bio"), a clinical stage biopharmaceutical company developing innovative biotherapeutics for metabolic diseases and cancers, today announces that DR10624, its first-in-class (FIC), tri-specific agonist targeting...
Everest Medicines Announces Completion of Patient Enrollment in Nefecon® China Open Label Extension Study
SHANGHAI, August 1, 2023 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK, "Everest", or the "Company"), a biopharmaceutical company focused on the development, manufacturing and commercialization of innovative medicines and vaccines, announced today the completion of patient enrollment for theChi...
Seegene unveils solutions to popularize molecular diagnostics at 2023 AACC
* Introduced syndromic quantitative PCR assays and a fully automated molecular diagnostic testing system, STARlet AIOS™ * Conducted symposium sessions on the usefulness of PCR testing in diagnosing gastrointestinal diseases * Participated in a presentation event for major Korean in-vitro dia...
Fosun Health Capital reached a cooperation agreement with Medicilon to explore innovative drug research and development together
BOSTON, July 31, 2023 /PRNewswire/ -- On July 25, 2023, Fosun Health Capital established by Fosun Pharma (stock code:600196.SH), reached a strategic partnership with Medicilon (stock code: 688202.SH). Meanwhile StarMab Biology (Suzhou) (jointly invested by the innovation drug research fund of Fo...
111 to Announce Second Quarter 2023 Unaudited Financial Results on August 24, 2023 - Conference Call to Follow
SHANGHAI, July 31, 2023 /PRNewswire/ -- 111, Inc. (NASDAQ: YI) ("111" or the "Company"), a leading tech-enabled healthcare platform company committed to digitally connecting patients with medicine and healthcare services in China, today announced that it will report its unaudited financial result...
Biosion Announces Phase II Study Start for Anti-TSLP mAb BSI-045B in Atopic Dermatitis (ADAMANT)
NEWARK, N.J. and NANJING, China, July 31, 2023 /PRNewswire/ -- Biosion USA, Inc. (Biosion), a global clinical-stage R&D biotechnology company, today announced the phase 2 study initiation for the evaluation of BSI-045B, an anti-TLSP mAb, in the treatment of atopic dermatitis (AD) . "We are pleas...
U.S. FDA Accepts Biologics License Application for GC Biopharma's GC5107B (Immune Globulin Intravenous (Human), 10% Liquid)
YONGIN, South Korea, July 31, 2023 /PRNewswire/ -- GC Biopharma (006280.KS), a global biopharmaceutical company dedicated to specialty plasma-derived therapeutics, today announced that the U.S. Food and Drug Administration (FDA) has accepted the Company's resubmission of the Biologics License App...
Transcenta Anti-sclerostin Monoclonal Antibody TST002 (Blosozumab) Received Approval from China CDE to Initiate Phase II Clinical Trial in Patients with Reduced Bone Mineral Density
SUZHOU, China, July 31, 2023 /PRNewswire/ -- Transcenta Holding Limited ("Transcenta") (HKEX: 06628), a clinical stage biopharmaceutical company with fully-integrated capabilities in discovery, research, development and manufacturing of antibody-based therapeutics, announces that it has receiv...
Daewoong Pharmaceutical submits a new drug application for Fexuprazan in China challenging the world's largest market for anti-ulcer drugs
– Daewoong Pharmaceutical, "Expecting the smooth approval with the successful phase 3 clinical trial inChina aiming to reach 100 countries by 2027" SEOUL, South Korea, July 31, 2023 /PRNewswire/ -- Daewoong Pharmaceutical's domestically produced drug no. 34 Fexuprazan, a newly developed one for t...
Latest Updates of 7 Viva's Portfolio Companies
HONG KONG, July 28, 2023 /PRNewswire/ -- In the face of ever-changing circumstances, technological innovation remains the eternal driving force behind the long-term growth of biopharmaceutical companies. It continuously breathes life into these enterprises, enabling them to successfully transitio...
HANQUYOU Marks 3rd Anniversary with Brilliant Achievements
* HANQUYOU is the China-developed biosimilar with the most marketing approvals, covering 41 countries and regions * The launch of HANQUYOU kicks off the competition between Chinese pharmaceutical companies and the world's bio-pharmaceutical companies in biosimilar monoclonal antibodies * He...
HANQUYOU Marks 3rd Anniversary with Brilliant Achievements
* HANQUYOU is the China-developed biosimilar with the most marketing approvals, covering 41 countries and regions * The launch of HANQUYOU kicks off the competition between Chinese pharmaceutical companies and the world's bio-pharmaceutical companies in biosimilar monoclonal antibodies * He...
Wuan Ankang Hospital's Innovative Tumor Treatment Attracts Many Patients
HANDAN, China, July 28, 2023 /PRNewswire/ -- In Handan City, Hebei Province, China, the Wu'an Ankang Hospital has 35-year of experience with tumor treatment. It has now become a second-class general hospital. The reason it attracts many patients from bothChina and abroad is that they practice hol...
GILEAD LAUNCHES GLOBAL ALL4LIVER GRANT PROGRAM TO HELP ACHIEVE THE WORLD HEALTH ORGANIZATION'S GOAL OF VIRAL HEPATITIS ELIMINATION BY 2030
– Gilead expands the ALL4LIVER Grant, Providing Grant Funding to Organizations inAfrica, South America, Asia and Oceania, Europe and North America – – Themed 'Test. Link. Prioritize', the Grant Program will Target Projects Focusing on Viral Hepatitis Elimination by 2030 – SINGAPORE, July 28, ...
Week's Top Stories
Most Reposted
ASTON MARTIN ARAMCO FORMULA ONE® TEAM ANNOUNCE PEPPERSTONE AS OFFICAL TRADING PARTNER
[Picked up by 308 media titles]
2025-01-07 09:00RuggON Unveils VORTEX Vehicle Mount Computer with AI-Enhanced, SATCOM-Ready
[Picked up by 306 media titles]
2025-01-06 21:00Brewing Connections and Spotlighting Café Culture: MIFB 2025 Hosts MNCC in Collaboration with MSCA
[Picked up by 305 media titles]
2025-01-06 14:00iWOW Unveils Age-Tech Innovations at CES 2025, Expanding Portfolio of IoT Solutions for Ageing-In-Place
[Picked up by 294 media titles]
2025-01-07 21:18CELEBRATE THE LUNAR NEW YEAR 2025 AT HOIANA RESORT & GOLF, WORLD'S LEADING FULLY INTEGRATED RESORT
[Picked up by 290 media titles]
2025-01-06 19:25