Pharmaceuticals
Seegene Showcased its Multiplex Technology at ADLM 2024
- Exhibited multiplex PCR testing technologies that provide comprehensive results from a single test - Presented high-multiplex assays and automated testing systems - Introduced the SG OneSystem™ business, the company's global technology-sharing initiative SEOUL, South Korea, Aug. 5, 2024 /P...
Antennova Announces CD73 Small Molecule Inhibitor Accepted for Mini Oral Presentation at ESMO Congress 2024
BOSTON, Aug. 5, 2024 /PRNewswire/ -- Antennova, a clinical-stage biotech company focused on oncology today announced that the orally administered CD73 small molecule inhibitor ATN-037(also known as ATG-037) has been accepted for Mini Oral presentation at the 2024 European Society of Medical Oncol...
QBIOTICS WELCOMES STEPHEN DOYLE AS CHIEF EXECUTIVE OFFICER
* Stephen Doyle has more than 24 years of experience in the global pharmaceutical industry, including leadership positions with companies such as Sanofi Aventis and Boehringer Ingelheim. He was most recently Chief Business Officer at Aslan Pharmaceuticals. * Mr Doyle brings extensive knowledg...
Formosa Pharmaceuticals Announces Licensing Agreement with Apotex Inc., for Commercialization of Clobetasol Propionate Ophthalmic Suspension for Ocular Surgery Relief and Recovery
TAIPEI, Aug. 5, 2024 /PRNewswire/ -- Taiwan-based Formosa Pharmaceuticals (" Formosa", 6838.TWO) announced today that the company has entered into an exclusive licensing agreement with Apotex Inc. ("Apotex"), for exclusive rights inCanada to the commercialization of clobetasol propionate ophthalmi...
DKSH and Kyowa Kirin Forge Strategic Partnership Across Asia-Pacific
DKSH has signed a strategic business partnership with Kyowa Kirin, a Japan -based global specialty pharmaceutical company, to provide comprehensive services for their specialty drugs in South Korea, Taiwan region, Singapore, Thailand, Malaysia and Hong Kong & Macau SAR. With DKSH's expertise, dis...
Innovent and SanegeneBio Announce First Participant Dosed in a Phase 1 Clinical Trial of IBI3016 (AGT siRNA)
SAN FRANSCISO and SUZHOU, China, Aug. 2, 2024 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, autoimmune, cardiovascular and metabolic,...
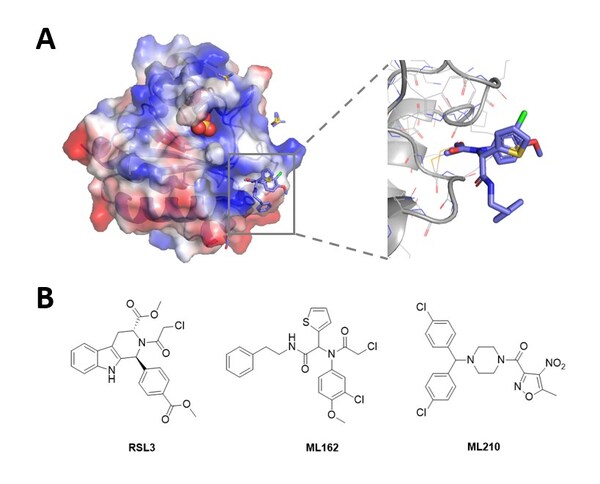
Novel Non-Covalent Hits Against GPX4 Identified Using the RiDYMO® Reinforced Dynamics Platform of DP Technology
Research on ferroptosis is gaining momentum, but the development of small molecule inhibitors faces numerous challenges BEIJING, Aug. 1, 2024 /PRNewswire/ -- Glutathione peroxidase 4 (GPX4) is recognized as a critical regulator of ferroptosis, playing a significant role in lipid and amino acid m...
Innovent Announces the NDA of Mazdutide for Type 2 Diabetes has been Accepted by the NMPA of China
SAN FRANCISCO, U.S. and SUZHOU, China, Aug. 1, 2024 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, autoimmune, cardiovascular and meta...
Biosyngen Partners with Singapore's Agency for Science, Technology and Research (A*STAR) to Advance Autoimmune Therapy
GUANGZHOU, China, Aug. 1, 2024 /PRNewswire/ -- Biosyngen has announced a strategic collaboration withSingapore's Agency for Science, Technology and Research (A*STAR) to enhance autoimmune therapy. The partnership was signed during the 14th Meeting of the Singapore-Guangdong Collaboration Council,...
WuXi Biologics' Four Manufacturing Facilities and Biosafety Testing Center Certified Again by European Medicines Agency for Ten Biologics
WUXI and SUZHOU, China, Aug. 1, 2024 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a leading global Contract Research, Development and Manufacturing Organization (CRDMO), today announced that four manufacturing facilities and Suzhou Biosafety Testing Center inChina have received Good Ma...
Sirnaomics Announces a Partnership with Gore Range Capital for Establishment of a JV, Sagesse Bio, to Advance Its RNAi Therapeutics into Aesthetic Medicine
HONG KONG, GERMANTOWN, Md. and SUZHOU, China, Aug. 1, 2024 /PRNewswire/ -- Sirnaomics Ltd.(the "Company", Stock Code: 2257, together with its subsidiaries, the "Group" or "Sirnaomics"), a leading biopharmaceutical company engaging in discovery and development of advanced RNAi therapeutics, today ...
Sirnaomics Announces a Partnership with Gore Range Capital for Establishment of a JV, Sagesse Bio, to Advance Its RNAi Therapeutics into Aesthetic Medicine
HONG KONG, GERMANTOWN, Md. and SUZHOU, China, Aug. 1, 2024 /PRNewswire/ -- Sirnaomics Ltd. (the "Company", Stock Code: 2257.HK, together with its subsidiaries, the "Group" or "Sirnaomics"), a leading biopharmaceutical company engaging in discovery and development of advanced RNAi therapeutics, to...
Prestige Biopharma's Herceptin Biosimilar Tuznue® Receives Positive CHMP Opinion from the EMA
* Following approval by the European Commission, Prestige Biopharma's Herceptin biosimilar Tuznue® would become the first biosimilar from a Singaporean company to be authorized in the European Union. SINGAPORE, Aug. 1, 2024 /PRNewswire/ -- Prestige Biopharma, a pioneer in biopharmaceuticals, a...
Medicilon and Hengrui Pharma Deepen Strategic Collaboration to Support Innovation in ADCs, Small Nucleic Acids, and CGT Drugs
BOSTON, July 31, 2024 /PRNewswire/ -- Recently, Shanghai Medicilon Inc. ("Medicilon") and Jiangsu Hengrui Pharmaceuticals Co., Ltd. ("Hengrui Pharma") reached a strategic collaboration agreement.The cooperative efforts will focus on preclinical evaluation of new drug modalities, particularly ADCs...
Kexing Biopharm's Albumin-bound Paclitaxel Granted EU Market Approval
SHENZHEN, China, July 31, 2024 /PRNewswire/ -- On July 28 2024, Kexing Biopharm announced European Commission approval of Apexelsin®, the generic drug to Bristol Myers Squibb's and Celgene's Abraxane®(Nab-paclitaxel). Apexelsin® is developed by WhiteOak Pharmaceutical B.V. and Kexing Biopharm is ...
JelloX Biotech collaborates with Mayo Clinic to develop AI enhanced 3D pathology imaging technology
HSINCHU, July 31, 2024 /PRNewswire/ -- JelloX Biotech Inc. ('JelloX') is pleased to announce that it has entered into a collaboration through a know-how agreement with Mayo Clinic to further develop and validate their 3D digital imaging and AI analysis technology. JelloX previously participated i...
FDA Grants Orphan Drug Designation (ODD) Status to Zymedi's ZMA001 for Pulmonary Arterial Hypertension
INCHEON, South Korea, July 30, 2024 /PRNewswire/ -- Zymedi (CEO Sunghoon Kim) announced that its first-in-class antibody treatment ZMA001, currently in development for pulmonary arterial hypertension (PAH), has been designated as an Orphan Drug by the U.S. Food and Drug Administration (FDA). Pul...
Porton Advanced and Geneseed Biotech Enter into Strategic Collaboration to Focus on Advancing circRNA Innovative Therapeutics
SUZHOU, China, July 30, 2024 /PRNewswire/ -- On July 29, 2024, Porton Advanced Solutions ("Porton Advanced") announced that it had reached a strategic cooperation with Guangzhou Geneseed Biotech Co., Ltd ("Geneseed Biotech"). Both sides will combine their respective strengths in the field of gene...
Connext Successfully Administers First Dose of CNT201 for Dupuytren's Contracture
World's First Genetically Recombinant Collagenase Therapeutics (CNT201) Begins Clinical Trials inAustralia SEOUL, South Korea, July 30, 2024 /PRNewswire/ -- Connext has announced the successful first administration of CNT201, its therapeutic treatment for Dupuytren's contracture, in patients. D...
NEURIM PHARMACEUTICALS RECEIVES POSITIVE CHMP OPINION ON SLENYTO® (PEDIATRIC PROLONGED-RELEASE MELATONIN) FOR THE TREATMENT OF INSOMNIA IN CHILDREN WITH NEUROGENETIC DISORDERS (NGDs)
TEL-AVIV, Israel, July 30, 2024 /PRNewswire/ -- Neurim Pharmaceuticals
Week's Top Stories
Most Reposted
Wonder Raises USD 12 Million Venture Debt from HSBC Innovation Banking to Drive Growth and Expansion
[Picked up by 322 media titles]
2026-02-02 10:00Agoda Launches Agoda Impact Lab at ASEAN Tourism Forum
[Picked up by 322 media titles]
2026-01-29 15:06AI adoption is widespread, but developer confidence is still catching up, Agoda report finds
[Picked up by 312 media titles]
2026-02-03 11:00Mastercard Launches Portfolio of Fleet Solutions in Asia Pacific
[Picked up by 308 media titles]
2026-02-04 09:00Colebrook Bosson Saunders Officially Launches Lana, A Circular Ergonomic Laptop Stand for the Hybrid Generation
[Picked up by 303 media titles]
2026-02-03 12:00