Pharmaceuticals
Ractigen Announces U.S. FDA Rare Pediatric Disease Designation (RPDD) Granted to RAG-18 for the treatment of Duchenne Muscular Dystrophy
SUZHOU, China, July 25, 2024 /PRNewswire/ -- Ractigen Therapeutics, a pioneering developer of small activating RNA (saRNA) therapeutics, today announced that the U.S. Food and Drug Administration (FDA) has granted Rare Pediatric Disease Designation (RPDD) to RAG-18, one of the company's lead saRN...
IASO Bio Announces NMPA Approval of IND Application for IASO-782 for Treatment of New Indication -- Systemic Lupus Erythematosus (SLE)
SHANGHAI, NANJING, China and SAN JOSE, Calif. , July 25, 2024 /PRNewswire/ -- IASO Biotechnology ("IASO Bio"), a biopharmaceutical company dedicated to discovering, developing, manufacturing and commercializing innovative cell therapy and antibody products, hereby announces thatthe investigation...
Australasian College of Pharmacy and MedAdvisor Solutions Launch MedAdvisor software training for the Queensland Community Pharmacy Scope of Practice Pilot
Uplifting pharmacists' role in primary care and positioning pharmacies as essential care destinations in local communities CAMBERWELL, Australia, July 25, 2024 /PRNewswire/ -- MedAdvisor Solutions and the Australasian College of Pharmacy today announced the launch of the MedAdvisor software tra...
Telix Successfully Prices A$650 Million Convertible Bonds
MELBOURNE, Australia, July 24, 2024 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX) (Telix, the Company, the Issuer) is pleased to announce that it has successfully pricedA$650 million 2.375 per cent convertible notes due 2029. The convertible notes, also referred to as "convertible bond...
Telix Announces A$600 Million Convertible Bonds Offering
MELBOURNE, Australia, July 23, 2024 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX) (Telix, the Company, the Issuer) today launches an offering ofA$600 million of convertible notes due 2029 (the Offering). The convertible notes, also referred to as "convertible bonds" (Convertible Bonds)...
Linical Named Best Global CRO by Global Health & Pharma Magazine
OSAKA, Japan, July 23, 2024 /PRNewswire/ -- Linical, a global Contract Research Organization (CRO) for full-service drug development, has been awarded the title ofBest Global CRO 2024 by Global Health & Pharma (GHP) Magazine. The award comes as part of the publication's annual Global Excellence A...
QDX Announces Collaboration with Prelude Therapeutics on Novel Oncology Programs
SINGAPORE, July 23, 2024 /PRNewswire/ -- QDX
Valinor Pharma Announces Acquisition by Grünenthal with a Total Deal Value of Approximately $250 Million
* Grünenthal acquires Valinor Pharma in an all-stock deal and becomes the owner of the global rights to Movantik® (naloxegol) * Valinor Pharma had owned Movantik since early 2023 and leveraged Apollo Care's commercial platform to grow Movantik volume in the U.S. while reducing gross-to-net a...
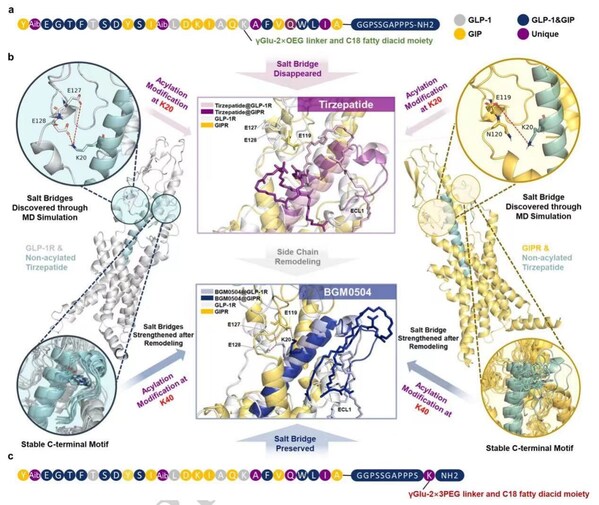
Nature Study Reveals: AI and Molecular Dynamics Designed BGM0504 Exhibits Superior Potency
SHANGHAI, July 22, 2024 /PRNewswire/ -- The molecular design strategy and experimental results of Bright Gene's dual GLP-1/GIP receptor agonist, BGM0504, have been published online in Scientific Reports, a sub-journal of Nature, on July 19, 2024. Bright Gene (Stock Code: 688166.SH) is an innovativ...
Gan & Lee Pharmaceuticals' Bi-weekly (twice a month) GLP-1 Receptor Agonist GZR18 Injection Achieved 17.29% Weight Loss at 30 Weeks in a Phase IIb Clinical Trial
* Obese and overweight participants receiving bi-weekly doses of 12 mg, 18 mg, 24 mg, 48 mg, and once-weekly dose of 24 mg GZR18 for 30 weeks achieved mean percent changes in body weight from baseline of -11.15%, -13.22%, -14.25%, -17.29%, and -17.78%, respectively, with the placebo group at -0...
Everest Medicines Announces First Chinese Patient Dosed in Global Phase 2b PALIZADE Trial of Zetomipzomib in Lupus Nephritis
SHANGHAI, July 22, 2024 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK, "Everest", or the "Company"), a biopharmaceutical company focused on the discovery, clinical development, manufacturing and commercialization of innovative therapeutics, today announced that the first Chinese patient has be...
Full-Life Technologies Announces the Appointment of Edward (Ted) Myles to Its Board of Directors
HEIDELBERG, Germany and GEMBLOUX, Belgium and SHANGHAI, China, July 21, 2024 /PRNewswire/ -- Full-Life Technologies, a fully integrated global radiotherapeutics company, today announcedthe appointment of Ted Myles to Full-Life's Board of Directors. Mr. Myles will serve as chairman of the audit c...
Innovent Announces the Second Phase 3 Trial of Mazdutide in Chinese Patients with Type 2 Diabetes Met Study Endpoints, and Plans to Submit NDA of Mazdutide to the NMPA
SAN FRANCISCO and SUZHOU, China, July 22, 2024 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, autoimmune, cardiovascular and metabolic, ...
Treatment of Type 2 diabetes with Chiglitazar combined with Metformin Approved for listing by the National Medical Products Administration
SHENZHEN, China, July 19, 2024 /PRNewswire/ -- On July 19, Shenzhen Chipscreen Biosciences Co., Ltd. (hereinafter referred to as "Chipscreen Biosciences") announced that it has received the Drug Registration Certificate approved and issued by the National Medical Products Administration (NMPA) fo...
Rona Therapeutics Raises $35 Million Series A+ Financing to Advance Innovative Metabolic siRNA Pipeline in Clinic and Next Generation RNA Platforms
* Financing led by LongRiver Investments with participation by new and existing global investment funds * Rona will use proceeds to progress leading metabolic siRNA pipeline programs into global development and expand extra-hepatic delivery platform in CNS and beyond SHANGHAI, July 19, 2024 /...
Harbour BioMed Announces Positive Profit Alert for 2024 Interim Results
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, July 18, 2024 /PRNewswire/ -- Harbour BioMed (the "Company"; HKEX: 02142), a global biopharmaceutical company committed to the discovery, development, and commercialization of novel antibody therapeutics focusing on oncology and immunol...
ACROBiosystems Launches GMP Brand Resilient Supply at ISSCR to Accelerate Cell and Gene Therapy Development
NEWARK, Del., July 18, 2024 /PRNewswire/ -- At the recent International Society for Stem Cell Research (ISSCR) Annual Meeting inHamburg, ACROBiosystems announced its new GMP-centered brand, Resilient Supply, with the mission of accelerating the advancement of cell and gene therapy (CGT) developme...
Assessment of supply chain risk key to improving medicine access
A four-year research project by INSEAD and five other institutions sheds light on how understandingmedical criticality, supply chain risk and their interactions could help us better address drug shortages. FONTAINEBLEAU, France, SINGAPORE and SAN FRANCISCO, July 18, 2024 /PRNewswire/ -- Drug s...
DX&VX is on Track and Accelerating the Development of Oral Obesity Treatment
* Confirmation of effects compared to global late-stage clinical trials * Accelerating the development of obesity treatment through early licensing out, global joint clinical trials, research funding investment, etc. SEOUL, South Korea, July 18, 2024 /PRNewswire/ -- DX&VX, which is gaining att...
Duoning showcases its comprehensive bioprocess solutions at Interphex Korea 2024
SHANGHAI, July 18, 2024 /PRNewswire/ -- Duoning Biotechnology Group ("Duoning"), a leading one-stop bioprocess solution provider, announced it has attended at Interphex Korea 2024 to showcase its total bioprocess solutions for the preparation of diverse biological products. Interphex 2024 provide...
Week's Top Stories
Most Reposted
Wonder Raises USD 12 Million Venture Debt from HSBC Innovation Banking to Drive Growth and Expansion
[Picked up by 322 media titles]
2026-02-02 10:00Agoda Launches Agoda Impact Lab at ASEAN Tourism Forum
[Picked up by 322 media titles]
2026-01-29 15:06AI adoption is widespread, but developer confidence is still catching up, Agoda report finds
[Picked up by 313 media titles]
2026-02-03 11:00Mastercard Launches Portfolio of Fleet Solutions in Asia Pacific
[Picked up by 310 media titles]
2026-02-04 09:00Colebrook Bosson Saunders Officially Launches Lana, A Circular Ergonomic Laptop Stand for the Hybrid Generation
[Picked up by 303 media titles]
2026-02-03 12:00