Pharmaceuticals
Harbour BioMed Announces Resubmission of Biologics License Application for Batoclimab to NMPA for Treatment of Generalized Myasthenia Gravis
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, June 26, 2024 /PRNewswire/ -- Harbour BioMed (the "Company"; HKEX: 02142), a global biopharmaceutical company committed to the discovery, development, and commercialization of novel antibody therapeutics focusing on oncology and immunol...
HUADONG MEDICINE Announces Positive Phase I Results for Innovative Oral Small Molecule GLP-1 Receptor Agonist HDM1002
HANGZHOU, China, June 26, 2024 /PRNewswire/ -- HUADONG MEDICINE, through its wholly-owned subsidiary Hangzhou Zhongmei HuaDong Pharmaceutical CO. LTD (Zhongmei HuaDong), has recently announced positive results from Phase I clinical trials of its innovative oral small molecule GLP-1 receptor agoni...
bioSeedin Expanded Global Licensing and Partnership Opportunities at 2024 BIO International Convention
NEWARK, Del., June 26, 2024 /PRNewswire/ -- bioSeedin, a flourishing global licensing and consulting platform, captivated attendees as an esteemed exhibitor at the BIO International Convention, the preeminent global biotech gathering. This prestigious event unfolded inSan Diego, spanning from Jun...
Positive Xanamem® biomarker trial published in the Journal of Alzheimer's Disease demonstrating potential Xanamem efficacy in patients with elevated blood pTau
The prospectively defined, double-blind analysis of biomarker-positive patients with mild Alzheimer's disease showed more rapid clinical progression in biomarker-positive patients, highlighting the suitability of elevated pTau for selecting patients in the on-going XanaMIA phase2b trial SYDNEY, ...
CARING PHARMACY UNVEILS MOM & CUTIE PRODUCT LINE IN CELEBRATORY 30TH ANNIVERSARY EVENT
KUALA LUMPUR, Malaysia, June 26, 2024 /PRNewswire/ -- CARiNG Pharmacy proudly celebrates its 30th anniversary with the grand launch of their 'Mom and Cutie' product line. To commemorate this milestone event, CARiNG Pharmacy held a Health & Beauty Carnival at 1 Utama Shopping Centre, Ground Floor ...
Fufang E'jiao Syrup's Breakthrough Research on Cancer-Related Fatigue Receives "Special Excellence Award" at 2024 ASCO Annual Meeting
CHICAGO, June 26, 2024 /PRNewswire/ -- The 2024 Annual Meeting of the American Society of Clinical Oncology (ASCO), a significant event in the global oncology community, was grandly held inChicago, USA, from May 31 to June 4, 2024. ASCO has announced the list of outstanding abstracts for 2024, wh...
Antengene Announces XPOVIO® (selinexor) National Health Insurance Service Approval for Reimbursement in South Korea
- XPOVIO® is the first XPO1 inhibitor approved for reimbursement by South Korea's National Health Insurance Service (NHIS) for the treatment of adult patients with relapsed/refractory multiple myeloma (R/R MM). - The approval of XPOVIO® by the NHIS in South Korea is the fourth national reimburse...
Simcere Zaiming Announce Approval of Cetuximab Beta in China by the NMPA
NANJING, China, June 26, 2024 /PRNewswire/ -- On June 25, 2024, Simcere Zaiming, an innovative oncology company and a subsidiary of Simcere Pharmaceutical Group (2096.HK), announced that Enlituo® (generic name: cetuximab beta injection), a new generation anti-epidermal growth factor receptor (EG...
Hyundai Bioscience succeeds in developing 'Multi-treatment for mosquito-borne viral infections' including Dengue Fever
* Developed a 'Niclosamide-based multi-treatment drug' that can simultaneously treat mosquito-borne viral infections such as four serotypes of dengue virus, Zika, Chikungunya, and Yellow Fever. * Accelerating preparations for dengue fever basket clinical trial scheduled to be conducted inBraz...
ONO PHARMA USA Announces Support for Conquer Cancer®, the ASCO Foundation
Conquer Cancer Supports Cancer Research and Education CAMBRIDGE, Mass., June 25, 2024 /PRNewswire/ -- ONO PHARMA USA today announced it will provide a sponsorship to Conquer Cancer®, the ASCO Foundation, with$1 million funding to advance cancer research and education. Conquer Cancer is a glob...
Porton Advanced introduces the MaxCyte ExPERT GTx Flow Electroporation instrument, continuing to provide customers with end-to-end cell and gene therapy CRO & CDMO services
SUZHOU, China, June 25, 2024 /PRNewswire/ -- Porton Advanced recently introduced the MaxCyte cGMP-grade ExPERT GTx Flow Electroporation instrument to its cell therapy platform, marking the company as the first cell therapy CDMO in China to possess this clinical-grade flow electroporation system. T...
CLL Community Urges Hospital Authority to List Next-Generation BTK Inhibitor on Drug Formulary to Bring Hope and Improve Quality of Life for CLL Patients
HONG KONG, June 25, 2024 /PRNewswire/ -- Chronic Lymphocytic Leukaemia ('CLL') is a lymphoproliferative disease characterised by the abnormal proliferation of lymphocytes. The progression of CLL is relatively slow and it is a mild haematological malignancy. Cypress Charitable Trust is aHong Kong ...
NX Group Signs Strategic Partnership Agreement with Controlant of Iceland
- Set to Provide Real-time Monitoring Service for Tracking Cargo Location and Strict Temperature Control - TOKYO, June 25, 2024 /PRNewswire/ -- NIPPON EXPRESS HOLDINGS, INC. has signed a strategic partnership agreement withIceland-based Controlant, Inc., a leading provider of real-time monitorin...
Innovent Presents the Results of the First Phase 3 Study of Mazdutide for Weight Management at the ADA's 84th Scientific Sessions
SAN FRANCISCO and SUZHOU, China, June 25, 2024 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, autoimmune, cardiovascular and metabolic, ...
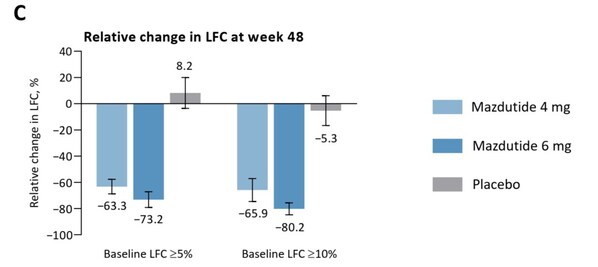
Innovent Announces Mazdutide Demonstrates 80.2% Reduction in Liver Fat Content in Exploratory Analysis of Phase 3 Weight Management GLORY-1 Study at ADA 2024
SAN FRANCISCO and SUZHOU, China, June 25, 2024 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, autoimmune, cardiovascular and metabolic, ...
TiumBio Presents Promising Clinical Data from Phase 1 of its Hemophilia Treatment Candidate TU7710 at ISTH 2024
* TU7710, long-acting recombinant activated factor VII, demonstrated a 5 to 7 times longer half-lifein a Phase 1 study than that of NovoSeven, a conventional hemophilia treatment for patients who develop inhibitors * TiumBio presented interim Phase 1a clinical data of TU7710 and discussed pot...
Juniper Biologics Expands Distribution Rights for Caris Life Sciences' Molecular Profiling in the Middle East and Africa
SINGAPORE, June 24, 2024 /PRNewswire/ -- Singapore-headquartered Juniper Biologics Pte Ltd (Juniper), a leading healthcare and pharmaceuticals company focused on commercialising novel therapies, has been granted distribution rights for Caris Life Sciences (Caris)' solid tumour molecular profiling...
Ascentage Pharma Announces Confidential Submission of Draft Registration Statement for Proposed Initial Public Offering of American Depositary Shares
ROCKVILLE, Md. and SUZHOU, China, June 24, 2024 /PRNewswire/ -- Ascentage Pharma (6855.HK) announced that it has confidentially submitted a draft registration statement on Form F-1 to the U.S. Securities and Exchange Commission (the "SEC") relating to the proposed initial public offering of Amer...
Gan & Lee Pharmaceuticals Announces Significant Progress on New Diabetes and Obesity Treatments at the American Diabetes Association's 84th Scientific Sessions
BEIJING and BRIDGEWATER, N.J., June 23, 2024 /PRNewswire/ -- Gan & Lee Pharmaceuticals (Gan & Lee, Shanghai Stock Exchange: 603087) announced the results of the Phase1b/2a clinical study of the Company's independently developed glucagon-like peptide-1 (GLP-1) receptor agonist, GZR18 Injection, in...
Sciwind Biosciences to Highlight Positive Results for Injectable Ecnoglutide (Phase 3), Oral Ecnoglutide (Phase 1), and Novel Amylin Analogs at the American Diabetes Association (ADA) 84th Annual Conference
* A Phase 1 study of oral ecnoglutide (1871-LB) showed it to be safe and well tolerated and result in pronounced weight loss (up to -6.76% after 6 weeks of dosing). Improved oral bioavailability enables a 15 to 30 mg daily dose of oral ecnoglutide to match or exceed the plasma exposure of weekl...
Week's Top Stories
Most Reposted
DBS is First Bank in Asia Pacific to Pilot Visa Intelligent Commerce for Everyday Payments
[Picked up by 319 media titles]
2026-02-16 10:00Marina Bay precinct partners UOB, Marina Bay Sands and Singapore Tourism Board, together with Disney Cruise Line, to illuminate Singapore's skyline with a fireworks sky show
[Picked up by 318 media titles]
2026-02-19 14:30Little Artists Art Studio, Singapore Shines at Art Capital 2026
[Picked up by 277 media titles]
2026-02-17 19:12Kung Fu Meets Spring -- Unitree Spring Festival Gala Robots Present "Cyber Real Kung Fu" in the Year of the Horse
[Picked up by 256 media titles]
2026-02-17 14:16SMU MBA Rises in FT Global Rankings, Excelling in ESG, Salary and Value-for-Money
[Picked up by 250 media titles]
2026-02-16 08:00