Pharmaceuticals
Ascentage Pharma Announces Closing of US$75 Million Equity Investment by Takeda
ROCKVILLE, Md. and SUZHOU, China, June 21, 2024 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancer, chronic hepatitis B (CHB), and age-related diseases, announced today that the agreed equity investment by Takeda has bee...
Keymed Biosciences Announces Safety and Efficacy Results of CM313 for Primary Immune Thrombocytopenia Published in NEJM
CHENGDU, China, June 20, 2024 /PRNewswire/ -- Keymed Biosciences Inc. (HKEX: 02162) today announced that Lei Zhang/Renchi Yang's team from the Institute of Hematology, Chinese Academy of Medical Sciences has recently published a research paper entitled "A Novel Anti-CD38 Monoclonal Antibody for T...
GC Cell Signs an MOU with PT Bifarma Adiluhung, an affiliate of KALBE group
* GC Cell and PT Bifarma Adiluhung announced a strategic partnership to
facilitate the entry of 'Immuncell-LC', the only approved autologous T-cell
therapy for HCC into the Indonesian market. YONGIN, South Korea, June 19, 2024
/PRNewswire/ --GC Cell
Golidocitinib Approved in China as First-in-class JAK1 Only Inhibitor for the Treatment of Relapsed or Refractory Peripheral T-Cell Lymphoma
* Golidocitinib is a first-in-class Janus kinase 1 (JAK1) only inhibitor approved for the treatment of r/r PTCL based on results from the multinational pivotal JACKPOT8B study. * Golidocitinib monotherapy demonstrated superior and durable clinical benefits and a favorable safety profile in r/...
GenFleet Receives IND Approval from China's NMPA for GFH375, an Oral KRAS G12D (ON/OFF) Inhibitor, in a Phase I/II Clinical Trial Treating Advanced Solid Tumor Patients with KRAS G12D Mutation
SHANGHAI, June 19, 2024 /PRNewswire/ -- GenFleet Therapeutics, a clinical-stage biotechnology company focusing on cutting-edge therapies in oncology and immunology, announcedChina's National Medical Products Administration (NMPA) has approved the clinical trial application for GFH375 (VS-7375) in...
Diabetes Researchers in China Demonstrate Early Insulin Therapy Significant Benefits Cardiovascular Health
BEIJING, June 19, 2024 /PRNewswire/ -- Recently, Professor Jianping Weng of the University of Science and Technology ofChina (USTC) and President of Anhui Medical University and his team of researchers from USTC, Southern Medical University and Peking University published the results of their lat...
Clarivate Identifies Seven Innovators in Antibody Drug Conjugates in New Companies to Watch Report
Emerging standouts set to transform drug discovery and development, revolutionize cancer treatment, and capture big pharma interest highlighted in third annual report LONDON, June 19, 2024 /PRNewswire/ -- Clarivate Plc (NYSE:CLVT), a leading global provider of transformative intelligence, has re...
Tang Prize in Biopharmaceutical Science Honoring Three Scientists
TAIPEI, June 19, 2024 /PRNewswire/ -- In a continuing series of laureate announcements, the Tang Prize Foundationtoday (June 19th) announced the 2024 Tang Prize in Biopharmaceutical Science recipients. The prestigious award has been jointly awarded toJoel F. Habener, Svetlana Mojsov, and Jens Juu...
Innovent Reports Oncology Pipeline Updates at Investor Meeting
SAN FRANCISCO and SUZHOU, China, June 19, 2024 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, metabolic, autoimmune, ophthalmology and o...
Menarini Asia-Pacific partners with Pharmacosmos to expand its reach of MonoFer® to Singapore and Malaysia
SINGAPORE, June 19, 2024 /PRNewswire/ -- Menarini Asia-Pacific (Menarini) today announced that it has expanded its partnership with Pharmacosmos A/S (Pharmacosmos), to include exclusive rights to commercialiseMonoFer® (ferric derisomaltose) in Singapore and Malaysia. This builds upon the successf...
YolTech and KACTUS Join Forces to Propel Innovative DNA editing system in Greater China
Strategic Partnership to Advance Gene Editing Technologies in Biomedical Applications SHANGHAI, June 18, 2024 /PRNewswire/ -- YolTech, a clinical-stage biopharmaceutical company specializing in mRNA-LNP delivery forin vivo gene editing therapies, has partnered with KACTUS, a leading developer se...
Clover Announces Positive Preliminary Phase Ⅰ Results for Bivalent RSV Vaccine Candidate SCB-1019 in Older Adults
-- Bivalent SCB-1019 significantly boosted RSV-A and RSV-B neutralizing antibody titers in older adults up to approximately 7,900 IU/mL (up to 8-fold increase) and approximately 46,700 IU/mL (up to 11-fold increase), respectively -- -- Favorable safety & reactogenicity profile comparable to sali...
Diplomatic Envoys to China Visit Fosun: Appreciating Oriental Lifestyle Aesthetics and Seeking Opportunities for Business Cooperation
SHANGHAI, June 18, 2024 /PRNewswire/ -- Over 40 guests, including 10 ambassadors toChina, 8 consuls general and other senior diplomats from 26 countries, visited Fosun on 14 June. The delegation toured along the Grand Yuyuan. From the intangible cultural heritage of the Old City of Shanghai to t...
Live from EHA 2024 | Ascentage Pharma Releases Updated Data of Lisaftoclax in Patients with R/R MM and AL Amyloidosis Highlighting Marked Improvement in ORR
SUZHOU, China and ROCKVILLE, Md., June 18, 2024 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancer, chronic hepatitis B (CHB), and age-related diseases, announced today that it has released updated data of the Bcl-2 inh...
Live from EHA 2024 | Posters Featuring Results from Three Studies of Olverembatinib, Including Encouraging Data from US Study in CML and Ph+ ALL
SUZHOU, China and ROCKVILLE, Md., June 18, 2024 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancer, chronic hepatitis B (CHB), and age-related diseases, announced today that updated results from three studies of olverem...
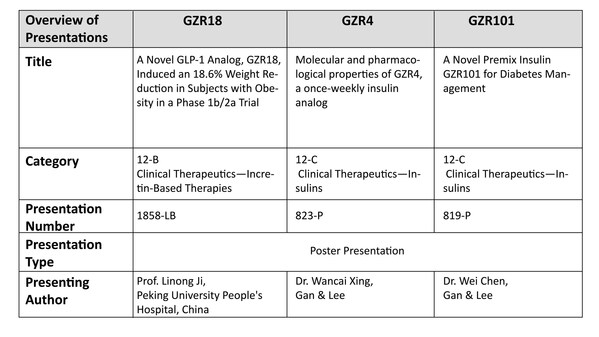
Gan & Lee Pharmaceuticals to Present Groundbreaking Data on Three Innovative Products at the American Diabetes Association's 84th Scientific Sessions
* A phase 1b/2a trial evaluated once- and bi-weekly GZR18, a novel GLP-1 analog, in Chinese subjects with obesity/overweight * Pre-clinical studies evaluated the molecular and pharmacological properties of GZR4, an investigational once-weekly insulin analog * Pre-clinical studies evaluated ...
ReviR Therapeutics Reaches Milestone in Novel Splicing Therapy Development in Collaboration with Asieris Pharmaceuticals
BRISBANE, Calif. and SHANGHAI, June 17, 2024 /PRNewswire/ -- ReviR Therapeutics, a biotechnology company specializing in the development of small molecule RNA splicing modulators for neurogenetic diseases and oncology indications, and Asieris Pharmaceuticals, a global biopharmaceutical leader in ...
Leads Biolabs Unveils Preclinical Data on LBL-047, a Novel, First-In-Class Long-Acting TACI/Anti-BDCA2 Bispecific Antibody Fusion Protein in an Oral Presentation at EULAR 2024 Congress
NANJING, China, June 17, 2024 /PRNewswire/ -- At the recent European League Against Rheumatism (EULAR) Congress inVienna, Austria, Nanjing Leads Biolabs Co., Ltd. (Leads Biolabs) delivered an oral presentation on its highly innovative therapeutic candidate LBL-047. LBL-047 is a first-in-class, l...
Faculty of Medicine Siriraj Hospital and Dhulikhel Hospital commemorate 15 years of collaborative relationship
BANGKOK, June 17, 2024 /PRNewswire/ -- In 2024, Thai-Nepalese bilateral relations reached a new milestone as the Faculty of Medicine Siriraj Hospital at Mahidol University inThailand and Dhulikhel Hospital at Kathmandu University inNepal celebrated the 15th anniversary of their esteemed partnersh...
IASO Bio Presented New Data of FUCASO® (Equecabtagene Autoleucel) for the Treatment of High-risk Newly Diagnosed Multiple Myeloma in Oral Presentation at EHA 2024
SHANGHAI and NANJING, China and SAN JOSE, Calif., June 15, 2024 /PRNewswire/ -- IASO biotechnology ("IASO Bio"), a biopharmaceutical company engaged in discovering, developing, manufacturing and marketing innovative cell therapies and antibody products, presented clinical data on the use of Equec...
Week's Top Stories
Most Reposted
DBS is First Bank in Asia Pacific to Pilot Visa Intelligent Commerce for Everyday Payments
[Picked up by 321 media titles]
2026-02-16 10:00Marina Bay precinct partners UOB, Marina Bay Sands and Singapore Tourism Board, together with Disney Cruise Line, to illuminate Singapore's skyline with a fireworks sky show
[Picked up by 317 media titles]
2026-02-19 14:30Little Artists Art Studio, Singapore Shines at Art Capital 2026
[Picked up by 277 media titles]
2026-02-17 19:12Tower Capital Asia announces majority investment in V-Key - a leader in digital identity and mobile application security
[Picked up by 265 media titles]
2026-02-13 01:52Appier Delivers Record Results Driven by Agentic AI Innovation
[Picked up by 263 media titles]
2026-02-13 17:03