Pharmaceuticals
Innovent Presents at the 2024 ASCO Annual Meeting on Clinical Data of anti-CLDN18.2 ADC (IBI343) in Patients with Advanced Pancreatic Cancer or Biliary Tract Cancer
SAN FRANCISCO, U.S. and SUZHOU, China, June 2, 2024 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, cardiovascular and metabolic, autoi...
GenFleet Therapeutics Announces Efficacy & Safety Result from Phase II Trial for First-line NSCLC Treatment in KROCUS Study, fulzerasib (KRAS G12C Inhibitor) in Combination with cetuximab, in a Late-breaking Abstract at the Oral Presentation of 2024 ASCO Annual Meeting
SHANGHAI and CHICAGO, June 1, 2024 /PRNewswire/ -- GenFleet Therapeutics, a clinical-stage biotechnology company focusing on cutting-edge therapies in oncology and immunology, today announced the phase II trial data of KROCUS Study, fulzerasib (GFH925, KRAS G12C inhibitor) in combination with cet...
Immunofoco Biotech to Unveil Solid Tumor CAR-T Programs Clinical Trial Data at 2024 ASCO Meeting
CHICAGO, June 1, 2024 /PRNewswire/ -- On June 1st, 2024, Immunofoco Biotech, a company dedicated to developing cell therapy products for solid tumors, announced that the clinical research data for two of its products have been accepted for presentation at the 2024 American Society of Clinical Onc...
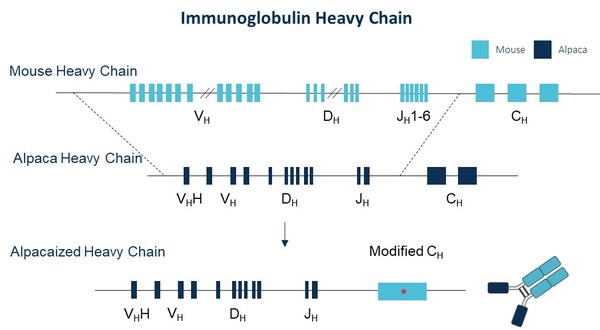
Immunocan Launches Innovative Alpaca-derived Nanobody Discovery Platform: ImmuAlpaca® Mouse
BOSTON, June 1, 2024 /PRNewswire/ -- Immunocan announces significant progress
in the construction of its alpaca-derived nanobody discovery platform,
ImmuAlpaca® mouse. The IGH-VDJ genes in the mouse genome have been successfully
replaced by alpaca IGH-VDJ genes in-situ.
TiumBio Presents Phase 1b Interim Results for TU2218 in Combination with Pembrolizumab at ASCO 2024 Annual Meeting
* Poster presentation features the interim data from an ongoing Phase 1b clinical trial of TU2218 plus Keytruda combination therapy in patients with advanced solid tumors * A 40% overall response rate (ORR) and 100% disease control rate (DCR) were achieved in high-dose cohort (195 mg/day) *...
Ivonescimab in Combination with Chemotherapy Approved in China by NMPA for 2L+ EGFRm NSCLC based on HARMONi-A Clinical Trial: Positive Trend Observed in Overall Survival towards Ivonescimab Plus Chemotherapy
Separate & Distinct from HARMONi-2 Announcement, HARMONi-A Showed Clinically Meaningful and Statistically Significant Benefit: PFS Hazard Ratio of 0.46 For Subset of Patients Previously Receiving 3rd Generation EGFR-TKI: PFS Hazard Ratio of 0.48 5.6% Treatment Discontinuation of Ivonescimab due...
GRIT and Quangang Forge Strategic Partnership to Accelerate Localization of Interleukin-2
SHANGHAI, May 31, 2024 /PRNewswire/ -- Shanghai Grit Biotechnology Co., Ltd. (GRIT) and Shandong Quangang Pharmaceutical Co., Ltd. (Quangang) announced the establishment of a formal strategic partnership to leverage both parties' R&D capabilities in innovative T-cell therapy. The objective is to ...
Neurophet to participate BIO USA… exploring global partnership and business
* Neurophet's AI brain image analysis technology improves time and cost efficiency for drug development * Aims to create business opportunities with global pharmaceutical companies SEOUL, South Korea, May 31, 2024 /PRNewswire/ -- Neurophet, an artificial intelligence (AI) solution company for...
SIFI receives positive CHMP opinion for AKANTIOR® (polihexanide 0.08%) in acanthamoeba keratitis
ACI SANT'ANTONIO, Italy, May 31, 2024 /PRNewswire/ -- SIFI announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has adopted a positive opinion recommending approval of AKANTIOR® (polihexanide under the international non-proprietary name),...
Record numbers expected at CPHI & PMEC China 2024 as Asian markets boom
Domestic and international markets in Asia surging as CPHI & PMEC China
welcomes 60,000 attendees and 3500 exhibitors
SHANGHAI and AMSTERDAM, May 31, 2024 /PRNewswire/ -- CPHI & PMEC China
Telix Announces Positive rPFS Data from ProstACT SELECT Trial of TLX591 rADC Therapy Candidate in Prostate Cancer
* TLX591 is an investigational anti-PSMA[1] radio-antibody-drug conjugate (rADC) therapy being developed for the treatment of mCRPC, differentiated by a short two-week dosing regimen. * Reported median radiographic progression-free survival (rPFS) is 8.8 months. * Builds on prior data from th...
LumaCina announces availability of Oral Morphine pain medication for New Zealanders
PERTH, Australia and MIAMI, May 31, 2024 /PRNewswire/ -- LumaCina, the pharmaceutical supplier and marketing division ofBridgewest Perth Pharma announce new production of Oral Morphine to meet critical drug shortage inNew Zealand. The shortfall of Oral Morphine has become increasingly concerning...
Teikoku Pharma USA, Inc. Announces NMPA Approved Lidoderm® in China on May 8, 2024
SAN JOSE, Calif., May 30, 2024 /PRNewswire/ -- Teikoku Pharma USA (TPU) announced today that the NMPA (National Medical Products Administration) has approved Lidoderm® ("lidocaine 5% patch as lidocaine cataplasms") for the treatment of Post Herpetic Neuralgia (PHN) inChina. TPU entered into an e...
OBiO Technology Officially Launched the Center for Clinical Evaluation and Translation of Advanced Therapies for Pediatric Rare and Genetic Diseases
SHANGHAI, May 30, 2024 /PRNewswire/ -- On May 23, OBiO Technology, a world leading contract development and manufacturing organization for cell and gene therapy, officially launched the "Center for Clinical Evaluation and Translation of Advanced Therapies for Pediatric Rare and Genetic Diseases" ...
Preliminary Clinical Data on Relma-Cel Injection in Adults with Active Systemic Lupus Erythematosus in China at the Eular 2024 Congress
SHANGHAI, May 30, 2024 /PRNewswire/ -- JW (Cayman) Therapeutics Co. Ltd (the "Company" or "JW Therapeutics", together with its subsidiaries, the "Group"), an independent and innovative biotechnology company focused on developing, manufacturing and commercializing cell immunotherapy products, pres...
Kexing Biopharm Obtained Clinical Trial Approval for its Self-developed Class I Innovative Drug--Long-acting Growth Hormone
SHENZHEN, China, May 30, 2024 /PRNewswire/ -- On the 24th of May, Kexing Biopharm (688136.SH) announced that Shenzhen Kexing Pharmaceutical Co., Ltd., its wholly-owned subsidiary, recently received a Notice of Approval for Drug Clinical Trials from the National Medical Products Administration, ap...
Full-Life Technologies Announces Clearance from FDA of IND Application for 225Ac-FL-020 for the Treatment of Metastatic Castration-Resistant Prostate Cancer
HEIDELBERG, Germany , May 29, 2024 /PRNewswire/ -- Full-Life Technologies (Full-Life), a fully integrated global radiotherapeutics company, today announced it has received clearance of its Investigational New Drug (IND) Application from the U.S. Food and Drug Administration (FDA) for clinical tr...
Soterios Pharma Announces Positive Topline Results from Phase II Study of STS-01 in the Treatment of Mild / Moderate Alopecia Areata
* Once-daily topical treatment of mild / moderate alopecia areata (AA) with 1% STS-01 met the primary efficacy endpoint of >30% Severity of Alopecia Tool (SALT) score improvement compared to patients receiving placebo (p<0.0096) * Multiple secondary endpoints met with significant total hair re...
AVELOS THERAPEUTICS ANNOUNCES KRW 17 BILLION SERIES B FUNDING ROUND, SURPASSING KRW 30 BILLION TOTAL RAISED
Stassets Investment Leads Round with Major Contribution, Joined by LSK Investment and Shinhan Capital Investment Provides Crucial Resources for Company's Focus on Developing Small Molecule Synthetic New Drugs in Synthetic Lethality, DNA Damage Response, and Cell Cycle Sectors First-In-Class AD1...
Veritas Genetics at the forefront of genomic screening with new studies presented at the European Society of Human Genetics Conference - ESHG 2024
* Veritas will present two pioneering studies at the European Society of Human Genetics Conference, inBerlin from June 1 to June 4. With a focus on elective genomic screening and newborn genomic screening, Veritas aims to set new standards in preventive medicine. * The Conference, now in its...
Week's Top Stories
Most Reposted
DBS is First Bank in Asia Pacific to Pilot Visa Intelligent Commerce for Everyday Payments
[Picked up by 319 media titles]
2026-02-16 10:00Marina Bay precinct partners UOB, Marina Bay Sands and Singapore Tourism Board, together with Disney Cruise Line, to illuminate Singapore's skyline with a fireworks sky show
[Picked up by 318 media titles]
2026-02-19 14:30Little Artists Art Studio, Singapore Shines at Art Capital 2026
[Picked up by 277 media titles]
2026-02-17 19:12Kung Fu Meets Spring -- Unitree Spring Festival Gala Robots Present "Cyber Real Kung Fu" in the Year of the Horse
[Picked up by 256 media titles]
2026-02-17 14:16SMU MBA Rises in FT Global Rankings, Excelling in ESG, Salary and Value-for-Money
[Picked up by 250 media titles]
2026-02-16 08:00