Pharmaceuticals
YS Biopharma to Hold Extraordinary General Meeting on May 21, 2024 and Announces the Appointment of Interim Chief Executive Officer
GAITHERSBURG, Md., May 8, 2024 /PRNewswire/ -- YS Biopharma Co., Ltd. (Nasdaq: YS) ("YS Biopharma" or the "Company"), a global biopharmaceutical company dedicated to discovering, developing, manufacturing, and delivering new generations of vaccines and therapeutic biologics for infectious disease...
FDA Grants Orphan Drug Designation to 9MW2821
SHANGHAI, May 7, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovation-driven biopharmaceutical company with entire industry chain, announced that its self-developed novel Nectin-4-targeting ADC (R&D code: 9MW2821) has been granted Orphan Drug Designation (ODD) by the U.S. Food and Drug Adminis...
Meihua International Medical Technologies Co., Ltd. to Present at the Emerging Growth Conference on May 9, 2024
Meihua International Medical Technologies Co., Ltd. invites individual and institutional investors as well as advisors and analysts, to attend its real-time, interactive presentation at the Emerging Growth Conference. YANGZHOU, China, May 7, 2024 /PRNewswire/ -- Meihua International Medical Tech...
Sciwind Biosciences and HK inno.N Corporation Announce Licensing and Partnership Agreement for Ecnoglutide Injection (XW003) in South Korea
HANGZHOU, China and SEOUL, South Korea, May 7, 2024 /PRNewswire/ -- Sciwind Biosciences Co., Ltd., a clinical stage biopharmaceutical company focused on developing treatments for metabolic disease, and HK inno.N Corporation, a South Korean commercial stage pharmaceutical company (KOSDAQ: 195940),...
GC Genome to Present New Clinical Data on Colorectal Cancer Detection at the ASCO Annual Meeting 2024
YONGIN, South Korea, May 7, 2024 /PRNewswire/ -- GC Genome Corporation, a leading diagnostics company, today announced that it will present the new clinical data of its AI-based liquid biopsy platform on colorectal cancer detection at the 2024 American Society of Clinical Oncology (ASCO) Annual ...
Innovent Receives NMPA Breakthrough Therapy Designation for IBI343(Anti-Claudin18.2 ADC)as Monotherapy for Advanced Gastric Cancer
SAN FRANCISCO and SUZHOU, China, May 7, 2024 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, cardiovascular and metabolic, autoimmune, ...
Alphamab Oncology to Present Chinese Clinical Data of JSKN003 for the Treatment of HER2-expressing Solid Tumors for the First Time at the 2024 ASCO Annual Meeting
SUZHOU, China, May 6, 2024 /PRNewswire/ -- Alphamab Oncology (stock code: 9966.HK) announced that data from the clinical study conducted inChina (JSKN003-102) of anti-HER2 bispecific antibody-drug conjugate (ADC) JSKN003 for the treatment of HER2-expressing advanced solid tumors, will be presente...
VYNDAMAX® (tafamidis) PBS-listed for adult patients with wild-type or hereditary transthyretin amyloid cardiomyopathy (ATTR-CM) with New York Heart Association (NYHA) Class I-II heart failure
* VYNDAMAX has been listed on the Pharmaceutical Benefits Scheme (PBS) for adult patients with wild-type or hereditary transthyretin amyloid cardiomyopathy (ATTR-CM)with New York Heart Association (NYHA) Class I-II heart failure.[1] * ATTR-CM is a debilitating and often fatal condition that l...
HanAll Biopharma Announces Initiation of Phase III Randomized, Double-Masked Vehicle Controlled VELOS-4 Trial Evaluating Tanfanercept for Treatment of Dry Eye Disease
* HanAll has initiated a Phase III VELOS-4 study to evaluate the efficacy and safety of tanfanercept in dry eye based on the findings from the previous Phase III VELOS-3 study. * Tanfanercept demonstrated statistically significant improvement on the secondary outcome measure, Schirmer testing...
Everest Medicines Announces Hong Kong Department of Health Approval of Nefecon® for the Treatment for Primary IgA Nephropathy in Adult Patients
SHANGHAI, May 2, 2024 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK, "Everest", or the "Company") announced that the Hong Kong Department of Health had approved Nefecon® for the treatment of primary immunoglobulin A nephropathy (IgAN) in adults at risk of disease progression.Hong Kong marks the...
3Shape Unveils Unite 3rd Generation: Access TRIOS Scans Anywhere, Anytime
COPENHAGEN, Denmark, May 1, 2024 /PRNewswire/ -- 3Shape launches Unite 3rd Generation, allowing dental professionals to access and work with their digital impressions and patient cases anywhere, anytime, via any mobile, tablet (Android, iOS), or web device. This update enables seamless and secure...
ArisGlobal Helps Boehringer Ingelheim Transform Safety Signal Processing by Leveraging Latest LifeSphere Solutions
LifeSphere Clarity drives efficiencies and reinvents the way Boehringer Ingelheim's pharmaceutical operations approach safety signals BOSTON, May 1, 2024 /PRNewswire/ -- ArisGlobal, a market leader in Life Sciences technology and the creator of LifeSphere®, announced today that research-driven g...
Jacobio Pharma Announced its KRAS G12C inhibitor reached the primary endpoint
BEIJING and SHANGHAI and BOSTON, April 30, 2024 /PRNewswire/ -- Jacobio Pharma (1167.HK) announced that the data from the Phase II registrational study of the KRAS G12C inhibitor glecirasib were offically reported at the April ASCO Plenary Series, which was held online. Prof. Yuankai Shi, chief ...
Eluminex Biosciences Announces FDA Acceptance of Investigational New Drug (IND) Application for EB-105 - A Novel Trispecific Fusion Antibody for Diabetic Macular Edema (DME) - and Upcoming Scientific Presentations
SAN FRANCISCO and SUZHOU, China, April 30, 2024 /PRNewswire/ -- Eluminex Biosciences (Eluminex), a privately-held biotechnology company focused on the development of advanced protein therapeutics for vision-threatening diseases and dermal facial aesthetics announced the acceptance of their EB-105...
National Medical Products Administration (NMPA) Approves Chipscreen Bioscience's Chidamide (Epidaza) combined with R-CHOP for the treatment of diffuse large B-cell lymphoma
SHENZHEN, China, April 30, 2024 /PRNewswire/ -- Shenzhen Chipscreen Biosciences Co., Ltd. (Chipscreen Biosciences, Stock Symbol: 688321.SH) announced that the company's lead innovative product Chidamide (Epidaza®) , an oral subtype-selective histone deacetylase (HDAC) inhibitor, combined with R-C...
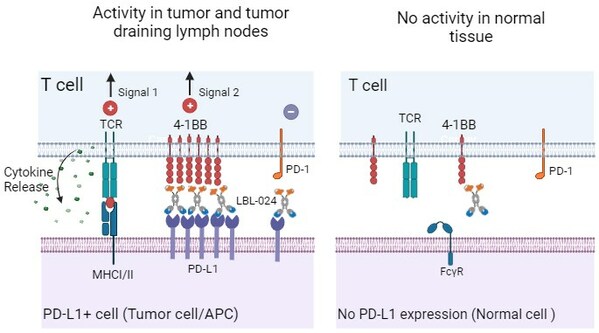
A Potential First-In-Class Drug: CDE Approved Single-Arm Pivotal Clinical Study of LBL-024, An Anti-PD-L1/4-1BB Bispecific Antibody Developed by Leads Biolabs
NANJING, China, April 30, 2024 /PRNewswire/ -- Nanjing Leads Biolabs Co., Ltd. (hereinafter referred to as "Leads Biolabs") announced that LBL-024, an anti-PD-L1/4-1BB bispecific antibody independently developed by Leads Biolabs with global intellectual property rights has received approval to co...
Medison Pharma and Alnylam Pharmaceuticals Announce Expansion of their Multi-Regional Partnership in Europe and Israel to Commercialize RNAi Therapeutics in additional LATAM and APAC markets including Australia
* The expanded partnership will allow Alnylam and Medison to help accelerate access for patients in multiple regions under one global alliance * Medison, the creator and leader of the multi-regional partnership category, will utilize its unique, unifiedplatform for efficient global commerciali...
HanAll Biopharma Reports Q1 2024 Financial Results and Provides Business Update
* Delivered solid performance to start 2024, with record-breaking first quarter revenue of34.1 billion KRW. Strong sales momentum continued from key products, funding investments in ongoing R&D programs. * Phase 3 VELOS-4 study of tanfanercept in dry eye disease expected to be initiated in th...
I-MAB Filed 2023 Annual Report on Form 20-F
ROCKVILLE, Md., April 30, 2024 /PRNewswire/ -- I-Mab (the "Company") (NASDAQ: IMAB), a U.S.-based, global biotech company, exclusively focused on the development and potential commercialization of highly differentiated immunotherapies for the treatment of cancer, today announced that it has filed...
111 to Announce First Quarter 2024 Unaudited Financial Results on May 23, 2024 - Conference Call to Follow
SHANGHAI, April 30, 2024 /PRNewswire/ -- 111, Inc. (NASDAQ: YI) ("111" or the "Company"), a leading tech-enabled healthcare platform company committed to digitally connecting patients with medicine and healthcare services inChina, today announced that it will report its unaudited financial result...
Week's Top Stories
Most Reposted
Marina Bay precinct partners UOB, Marina Bay Sands and Singapore Tourism Board, together with Disney Cruise Line, to illuminate Singapore's skyline with a fireworks sky show
[Picked up by 328 media titles]
2026-02-19 14:30Never Miss a Message: Agoda's Customer Support Now Travels With You
[Picked up by 327 media titles]
2026-02-24 12:00NextFin Asia: A New Dedicated Fund for the Catapult: Inclusion SE Asia Program to Further Scale Inclusive Finance Fintechs in ASEAN
[Picked up by 311 media titles]
2026-02-23 08:00Klook and Osaka Convention & Tourism Bureau sign MoU to advance inbound tourism and foster socio-economic development throughout Osaka Prefecture
[Picked up by 302 media titles]
2026-02-24 16:13Vitafoods Asia 2026 Expands by 30%: A Bigger, More Dynamic Trade Event with Exciting New Features & Increased International Participation
[Picked up by 288 media titles]
2026-02-23 10:09