Pharmaceuticals
Telix Signs Agreement to Acquire QSAM Biosciences and Its Bone Cancer Targeting Platform
MELBOURNE, Australia , Feb. 8, 2024 /PRNewswire/ --.Telix Pharmaceuticals Limited (ASX: TLX, Telix, the Company) today announces it has entered into an agreement to acquire QSAM Biosciences, Inc. (U.S. OTC: QSAM) and its lead investigational drug Samarium-153-DOTMP (153Sm-DOTMP). QSAM is a United...
Complete Genomics and seqWell Announce Codevelopment Partnership at AGBT
Companies will work together to offer fast, cost-effective
ultra-high-throughput library preparation workflows
SAN JOSE, Calif. and BEVERLY, Mass., Feb. 7, 2024 /PRNewswire/ --
Complete Genomics,
I-Mab Signs Agreement to Divest its Assets and Business Operations in China
* Agreement marks an important milestone to advance the Company's intent to become a U.S.-based biotech * Agreement provides for a strategic focus in advancing I-Mab's potential of differentiated oncology clinical assets and builds shareholder value by streamlining the operating model, reduc...
Innovent's First New Drug Application of Mazdutide for Chronic Weight Management has been Accepted by the NMPA of China
ROCKVILLE, Md. and SUZHOU, China, Feb. 7, 2024 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, autoimmune, cardiovascular and metabolic...
The Results of Phase II Clinical Study of KN046 in Combination with Nab-paclitaxel in TNBC were Published in Nature Communications
SUZHOU, China, Feb. 6, 2024 /PRNewswire/ -- Alphamab Oncology (stock code: 9966.HK) announced that the phase Ⅱclinical research results of anti- PD-L1/CTLA-4 bispecific antibody KN046 in combination with nab-paclitaxel as first-line treatment in advanced triple-negative breast cancer (TNBC) were ...
SMC Laboratories Reveals Innovative STAM™-HCC/IO+ Mouse for Identifying and Developing Novel Therapeutic Drugs for Liver Cancer, Blazing a Trail in the Immuno-Oncology Field
TOKYO, Feb. 7, 2024 /PRNewswire/ -- SMC Laboratories, Inc. ("SMC Laboratories"), a non-clinical contract research organization (CRO) that provides consulting and contract services for pharmacology studies utilizing technology and know-how related toMetabolic dysfunction associated steatohepatiti...
SMC Laboratories Reveals Innovative STAM™-HCC/IO+ Mouse for Identifying and Developing Novel Therapeutic Drugs for Liver Cancer, Blazing a Trail in the Immuno-Oncology Field
TOKYO, Feb. 7, 2024 /PRNewswire/ -- SMC Laboratories, Inc. ("SMC Laboratories"), a non-clinical contract research organization (CRO) that provides consulting and contract services for pharmacology studies utilizing technology and know-how related to Metabolic dysfunction associated steatohepatit...
Technoderma Medicines Phase 2 Clinical Trial of TDM-105795 Demonstrates Hair Growth in Androgenetic Alopecia
CHENGDU, China, Feb. 5, 2024 /PRNewswire/ -- Technoderma Medicines, Inc. ("the Company"), a clinical stage biopharmaceutical company, is pleased to report that the Company has completed its Phase 2a clinical trial (NCT05802173) of TDM-105795 topical solution for Androgenetic Alopecia (AGA). This ...
Luye Pharma Grants Myung In Pharm Exclusive Rights to Commercialize Rivastigmine Twice Weekly Transdermal Patch in South Korea
YANTAI, China, Feb. 5, 2024 /PRNewswire/ -- Luye Pharma Group has entered into an agreement with Myung In Pharm, granting the latter the exclusive rights to commercialize Rivastigmine Twice Weekly Transdermal Patch inSouth Korea. Developed by Luye Pharma, this drug is used to treat mild to modera...
CARiNG Pharmacy Awarded Gold in Esteemed Putra Aria Brand Awards 2023 in Retail Category
KUALA LUMPUR, Malaysia, Feb. 5, 2024 /PRNewswire/ -- CARiNG Pharmacy proudly announced its victory at the second edition of the prestigious Putra Aria Brand Awards 2023, securing the coveted Gold Award in the Retail Category in recognition of its outstanding contributions to the retail industry. ...
Celebrating a Legacy of Care - Pantai Hospital Kuala Lumpur Celebrates 50 Years of Excellence in Healthcare and Unveils Its Future Vision with Gala Dinner
KUALA LUMPUR, Malaysia, Feb. 5, 2024 /PRNewswire/ -- Pantai Hospital Kuala
Lumpur (PHKL) marked a significant milestone with its 50th Anniversary Gala
Dinner, an event that highlighted the hospital's impressive legacy and future
ambitions in the healthcare sector.
Innovent Announces Retirement of CFO and Appointment of New CFO
ROCKVILLE, Md. and SUZHOU, China, Feb. 5, 2024 /PRNewswire/ -- Innovent Biologics, Inc. ("c") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, autoimmune, metabolic, ophthalmology and other ...
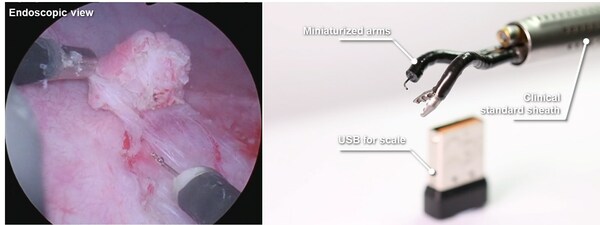
Agilis Robotics Unveils Groundbreaking Advancements in Endoluminal Surgery
HONG KONG, Feb. 2, 2024 /PRNewswire/ -- Agilis Robotics, a leading innovator in surgical robotics, is spearheading a paradigm shift in the field of "incisionless" endoluminal surgery. The company has made remarkable technological breakthroughs in developing highly miniaturized and flexible robot...
Acepodia Announces FDA Clearance of Investigational New Drug Application for ACE2016, a First-in-Class Allogeneic Anti-EGFR Cell Therapy
* ACE2016 Is an Allogeneic Gamma Delta 2 (γδ2) T Cell Therapy Targeting the Epidermal Growth Factor Receptor (EGFR) in Patients With Solid Tumors. * Phase 1 Clinical Study Expected to Begin in H2 2024. ALAMEDA, Calif. and TAIPEI, Feb. 4, 2024 /PRNewswire/ -- Acepodia (6976:TT), a clinical stag...
Blossoming Flowers at the Top of the Biopharmaceutical Tree -- Reflections on the Flourishing of Sanyou's 4C Business
SHANGHAI, Feb. 2, 2024 /PRNewswire/ -- In 1996, MDS Pharma Service invested in and established the first true Contract Research Organization (CRO) inChina, dedicated to clinical trial business for new drugs. This marked the official launch of the CRO industry inChina. The concept of CRO originat...
Lumosa Therapeutics and CHI Memorial announce new study for acute stroke
TAIPEI and CHATTANOOGA, Tenn., Feb. 2, 2024 /PRNewswire/ -- Lumosa Therapeutics (Lumosa; 6535.TWO), together with CHI Memorial have announced the initiation of a Phase2b clinical trial for LT3001, a groundbreaking drug for treating acute stroke. This new drug combines clot-busting and nerve-prote...
Inmagene Exercises Option to Obtain Exclusive Worldwide License for IMG-007 and IMG-004 from HUTCHMED
* Inmagene exercised its option under the previously announced collaboration agreement to obtain an exclusive, royalty-bearing license for IMG-007, an anti-OX40 monoclonal antibody (mAb) and IMG-004, a Bruton Tyrosine Kinase (BTK) inhibitor * Inmagene retains rights to develop and commerciali...
World Cancer Day 2024: RemeGen Announces Three Significant Developments in the Global Fight Against Cancer, Narrowing the Care Gap
YANTAI, China, Feb. 2, 2024 /PRNewswire/ -- RemeGen Co. Ltd.
Results of Phase II Study on Qilu Pharmaceutical's Novel Drug QL1706 Published in Signal Transduction and Targeted Therapy
JINAN, China, Feb. 2, 2024 /PRNewswire/ -- On January 29th, the results of the phase II study on Qilu Pharmaceutical's novel anticancer drug iparomlimab and tuvonralimab (QL1706) were published online inSignal Transduction and Targeted Therapy (Impact Factor=39.3). The study investigated the use ...
Fapon and Bio Farma Sign MOU for Medical Innovations
DONGGUAN, China, Feb. 2, 2024 /PRNewswire/ -- On February 1, 2024, Fapon, a global leading life science company, andBio Farma, a state-owned pharmaceutical holding company inIndonesia specializing in vaccines, life science products, and other pharmaceuticals, signed a memorandum of understanding ...
Week's Top Stories
Most Reposted
Visa partners with Laufey to spread the magic of travel in Asia Pacific; to be Official Payment Partner for Laufey: A Matter of Time Tour
[Picked up by 309 media titles]
2026-03-04 12:35SMU and Fudan Launch Region's First Tech-Focused DBA
[Picked up by 300 media titles]
2026-03-02 09:15Klook's Spring Readiness Index shows how Asia's travelers are preparing for spring travel across Japan, South Korea, and Mainland China
[Picked up by 290 media titles]
2026-03-03 15:49Infobip is set to launch AgentOS to orchestrate autonomous AI-driven customer journeys at scale
[Picked up by 290 media titles]
2026-03-02 09:00COL and NASDAQ-Listed BeLive Holdings Unveil World's First "Microdrama in a Box" in Headline Hong Kong FILMART 2026 Launch
[Picked up by 287 media titles]
2026-03-05 17:14