Pharmaceuticals
Johnson & Johnson Innovation announces extension of innovation partnering office at Monash University in collaboration with Victorian State Government to accelerate the life-sciences hub
- Victorian State Government extends financing of JJIPO@Monash for an additional two years - Extension will further boost Victorian hub for researchers and early-stage companies to connect with industry experts and deliver novel healthcare solutions MELBOURNE, Australia, July 15, 2021 /PRNewswi...
Ascentage Pharma and Innovent Biologics Reach Multifaceted Strategic Agreement Totaling US$245 Million Including Commercialization of Olverembatinib (HQP1351) in China, Joint Clinical Development of Lisaftoclax (APG-2575) and Equity Investment
SUZHOU, China, and ROCKVILLE, Md., July 14, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused biopharmaceutical company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, and Innovent Biologics (1801.HK, "Innovent"), today an...
Innovent and Ascentage Pharma Reach Multifaceted Strategic Agreement including Joint Commercialization of Olverembatinib in China, Joint Clinical Development on Multiple Products, and Equity Investment
SAN FRANCISCO and SUZHOU, China, July 14, 2021 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of cancer, metabolic, autoimmune and other major diseas...
Nippon Express Italia Acquires GDP Certification for Company-owned Warehouse near Milan Malpensa Airport
TOKYO, July 14, 2021 /PRNewswire/ -- Nippon Express Italia S.P.A. (hereinafter " NE Italy"), a local subsidiary of Nippon Express Co., Ltd., obtained Good Distribution Practice (GDP) certification from the certification body CERTIQUALITY S.r.l., effectiveMay 25, for its company-owned warehouse nea...
Kintor Pharmaceutical Receives IND Clearance by the U.S. FDA for GT20029 to Treat Androgenetic Alopecia and Acne
SUZHOU, China, July 13, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited (HKEX:9939), a clinical-stage biotechnology company developing innovative small-molecule and biological therapeutics, today announced that the U.S. Food and Drug Administration (FDA) has cleared an Investigational New Drug...
Seegene Introduces New SARS-CoV-2 Variants Detection Test That Can Screen Six Virus Variants Including the Delta and Delta Plus
* Seegene launches new multiplex PCR test capable of screening six SARS-CoV-2 variants including the Delta and Delta Plus variants attributable for a recent surge in global COVID-19 cases. * The new CE-IVD marked variants detection kit to differentiate 10 major COVID-19 variants including the...
Everest Medicines Announces Strategic Collaboration with Medbanks Health Technology to Develop Innovative Healthcare Service Solutions for Patients in China
SHANGHAI, July 13, 2021 /PRNewswire/ -- Everest Medicines
Ascletis Announces Dosing of the First Cohort of Healthy Subjects in the FXR Agonist ASC42 Bridging Study for Chronic Hepatitis B Indication in China
--This bridging study to select doses for the Phase II trial in China in patients with chronic hepatitis B (CHB) --ASC42 clinical trials are being conducted in the U.S. and China for non-alcoholic steatohepatitis (NASH) and CHB. HANGZHOU and SHAOXING, China, July 13, 2021 /PRNewswire/ -- A...
Servier and Nymirum Announce Strategic Collaboration to Discover and Develop RNA-Targeted Small Molecule Therapeutics
PARIS, July 12, 2021 /PRNewswire/ -- Servier, a global independent pharmaceutical Group, and Nymirum, a pioneer in RNA-targeted small molecules, announced todaythat they have entered into a strategic collaboration to identify and develop RNA-modulatory drugs for the treatment of neurological ...
Hummingbird Bioscience and MD Anderson Announce Strategic Research Collaboration To Advance Innovative Immunotherapies
HOUSTON and SINGAPORE, July 12, 2021 /PRNewswire/ -- Hummingbird Bioscience, an innovative clinical-stage biotech company focused on developing precision therapies against hard-to-drug targets to produce major improvements in treatment outcomes, andThe University of Texas MD Anderson Cancer Ce...
Stem cell therapy company Amniotics raises SEK 60 million before deduction of transaction costs, in First North IPO to advance lead candidate PulmoStem(TM) into clinic
Novel source of stem cells in full-term amniotic fluid provides basis for broad pipeline LUND, Sweden, July 12, 2021 /PRNewswire/ -- Amniotics AB (publ) ("Amniotics" or the "Company"), a stem cell therapy company has raisedSEK60 million through its recent listing on Nasdaq First North Growth Mar...
Everest Medicines Announces Strategic Commercial Partnership with Global Technology Company, Tencent
SHANGHAI, July 12, 2021 /PRNewswire/ -- Everest Medicines
Innovent Announces the First Patient Dosed in the Phase 1 Study of IBI323 (Anti-LAG-3/PD-L1 Bispecific Antibody) in Patients with Advanced Malignant Tumors
SAN FRANCISCO and SUZHOU, China, July 12, 2021 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of cancer, metabolic, autoimmune and other major diseas...
Kintor Pharmaceutical Announced FDA Has Greenlighted Pyrilutamide's Phase II Clinical Trial for Androgenetic Alopecia in the US
SUZHOU, China, July 11, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited (HKEX:9939), a clinical-stage biotechnology company developing innovative small molecule and biological therapeutics, today announced that the U.S. Food and Drug Administration (FDA) has greenlighted pyrilutamide's phase...
Clean interim safety review of RhoVac's clinical phase IIb study in prostate cancer
STOCKHOLM, July 9, 2021 /PRNewswire/ -- RhoVac AB ("RhoVac"), a Swedish cancer immunotherapy company, announces today onJuly 9th 2021, that its Safety Monitoring Committee has conducted a planned interim safety review of its clinical phase IIb trial in prostate cancer, known as BRaVac. The safety...
I-Mab Expands Emerging Portfolio of Next Generation Novel Oncology Therapeutics Through Cutting-Edge mRNA and AI Technology Platforms
SHANGHAI and GAITHERSBURG, Md., July 9, 2021 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical stage biopharmaceutical company committed to the discovery, development and commercialization of novel biologics, today announced the signing of two new collaborations with emerging biote...
Sequanta and Mission Bio announce strategic partnership
SHANGHAI, July 8, 2021 /PRNewswire/ -- Sequanta Technologies Co., Ltd. ("Sequanta") and Mission Bio. ("Mission Bio") are pleased to announce that they have entered into a strategic partnership, providing high-quality single-cell sequence services using Mission Bio's products to customers in mainl...
Innovent Announces NMPA Acceptance of New Drug Application for the FGFR1/2/3 Inhibitor (Pemigatinib) for the Treatment of Adults with Previously Treated, Unresectable Locally Advanced or Metastatic Cholangiocarcinoma with a FGFR2 Fusion or Rearrangement
SAN FRANCISCO, and SUZHOU, China, July 9, 2021 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of cancer, metabolic, autoimmune and other major diseas...
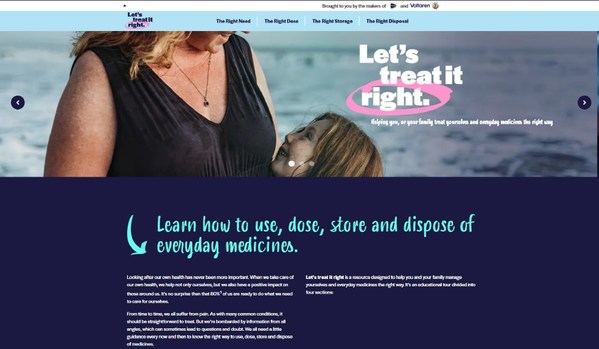
COVID pandemic heightens a lack of confidence amongst the public about which medicines to take and how to use them
New campaign, Let's treat it right, launches as online searches for self-care surge globally[1], as people take more responsibility for their everyday health. NYON, Switzerland, July 8, 2021 /PRNewswire/ -- Today sees the launch of Let's treat it right, a major new health education campaign d...
Nippon Express (India) Newly Acquires GDP Certification at Three Locations
TOKYO, July 8, 2021 /PRNewswire/ -- Nippon Express (India) Private Limited (hereinafter "NE India"), a local subsidiary of Nippon Express Co., Ltd., obtained Good Distribution Practice (GDP) certification in April for its temperature-controlled hubs inDelhi, Mumbai and Ahmedabad, evidencing the ...
Week's Top Stories
Most Reposted
AVPN's AI Opportunity Fund Expands Regional Efforts to Build AI Skilling Infrastructure for a Future-Ready Workforce Across Asia-Pacific
[Picked up by 302 media titles]
2026-01-21 12:39Government of Telangana and Blaize Sign MoU at Davos to Launch Telangana AI Innovation Hub and Advance Applied AI Initiatives
[Picked up by 297 media titles]
2026-01-22 15:16Infobip strengthens leadership to drive growth and innovation
[Picked up by 282 media titles]
2026-01-16 11:00Klook's study of 374,000 reviews finds that lesser-known cities create deeper emotional connection with travelers
[Picked up by 280 media titles]
2026-01-20 15:40Novo Holdings Invests in Surya Hospitals to Strengthen Access to High-Quality Women's and Children's Healthcare in India
[Picked up by 277 media titles]
2026-01-20 09:00