Pharmaceuticals
Ildong Pharmaceutical starts Phase 1 study of new diabetes therapy in Germany
SEOUL, South Korea, July 1, 2021 /PRNewswire/ -- A Phase 1 study on new drug candidate IDG16177 of novel oral treatment for type 2 diabetes, which is being developed by Ildong Pharmaceutical, is initiated inGermany. On June 28th (local time), Ildong Pharmaceutical obtained approval for the Phas...
I-Mab Announces Multiple Advancements of 4-1BB Bispecific Antibody Portfolio
* First patient dosed in U.S. phase 1 clinical trial of TJ-CD4B/ABL111 in patients with advanced or metastatic solid tumors * China sites to join the dose expansion part of the study to accelerate TJ- CD4B/ABL111 development * Results of TJ-L14B/ABL503 preclinical studies accepted for publica...
Alterity Therapeutics granted a new US patent targeting major neurodegenerative diseases including Alzheimer's and Parkinson's
MELBOURNE, Australia and SAN FRANCISCO, July 1, 2021 /PRNewswire/ -- Alterity Therapeutics (ASX: ATH, NASDAQ: ATHE) ("Alterity" or "the Company") has today announced the granting of a new composition of matter patent by the United States Patent and Trademark Office (USPTO). The patent secures a ...
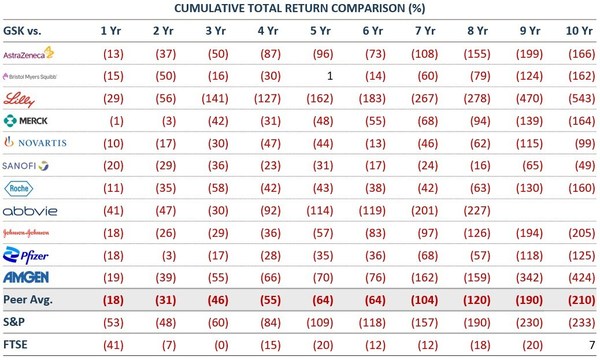
Elliott Publishes Letter on GlaxoSmithKline
Elliott believes GSK has a substantial value creation opportunity – 45% upside in its share price – ahead of Consumer Health separation and greater beyond Despite its strengths, GSK has a poor record of operational execution and value creation, leading to scepticism about the Company's future, an...
Kazia Provides Progress Update on Paxalisib and EVT801 Clinical Programs
SYDNEY, June 30, 2021 /PRNewswire/ -- Kazia Therapeutics Limited (NASDAQ: KZIA; ASX: KZA), an oncology-focused drug development company, is pleased to provide an update on recent progress with its two pipeline assets, paxalisib and EVT801. Key Points * GBM AGILE pivotal study of paxalisib is...
I-Mab Honored with Top Rankings by Institutional Investor
SHANGHAI and GAITHERSBURG, Md., June 30, 2021 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical stage biopharmaceutical company committed to the discovery, development and commercialization of novel biologics, today announced that it was ranked among the top companies by leading gl...
Antheia Announces $73M Series B to Commercialize Synthetic Biology Platform for Essential Medicines
Led by Viking Global Investors, the Series B round will be used to bring Antheia's first pharmaceutical compounds to market, expand its pipeline and scale production in global markets MENLO PARK, Calif., June 30, 2021 /PRNewswire/ -- Antheia, a synthetic biology company enabling next-generation ...
Akeso Awarded as 2021 "Most Honored Company" by Institutional Investor
HONG KONG, June 30, 2021 /PRNewswire/ -- Akeso, Inc. ("Akeso" or the "Company", stock code: 9926.HK) is pleased to announce today that, Akesohas been awarded as "Most Honored Company" in the "2021 All-Asia Executive Team" survey published byInstitutional Investor, and has been ranked top 3 in all...
Clover and Dynavax Announce Commercial Supply Agreement of Dynavax's CpG 1018 Adjuvant for Clover's COVID-19 Vaccine Candidate
* Clover to procure CpG 1018 adjuvant from Dynavax for use in the commercial production of Clover's COVID-19 vaccine candidate, SCB-2019 (CpG 1018/Alum) * Pending conditional regulatory approvals, Clover expects to commence product launch of SCB-2019 (CpG 1018/Alum) by the end of 2021 CHENGDU,...
Clover Announces Advance Purchase Agreement with Gavi for Over 400 Million Doses of Clover's COVID-19 Vaccine for the COVAX Facility
* Pending WHO EUL for its COVID-19 vaccine candidate, Clover is committed to providing 64 million doses in 2021 and Gavi has options for an additional 350 million doses in 2022 * Clover will receive a significant upfront payment, a payment upon positive Phase 2/3 data, potential payments upon...
Envirotainer Greets China Airlines as the latest airline to approve the new Releye® RLP container
STOCKHOLM, June 30, 2021 /PRNewswire/ -- Envirotainer, the global market leader in secure cold chain solutions for air transportation of pharmaceuticals, today announced that China Airlines has approved the Envirotainer Releye® RLP for usage on their aircrafts. With this approval, China Airlines ...
OnCusp Therapeutics Commences Operations to Advance Innovative Oncology Therapies
NEW YORK, June 29, 2021 /PRNewswire/ -- OnCusp Therapeutics (or "OnCusp"), a global biopharmaceutical company focused on translating cutting-edge research assets into innovative oncology therapeutics, announced that it has officially commenced operations both inNew York and Shanghai. OnCusp succ...
European Organisation for Research and Treatment of Cancer (EORTC) and Pierre Fabre Partner to Address Treatment Gap for Stage 2 Melanoma Patients
Partnership builds on complementary strengths to advance research in melanoma with a focus on Stage 2 melanoma patients BRUSSELS and CASTRES, France, June 29, 2021 /PRNewswire/ -- European Organisation for Research and Treatment of Cancer (EORTC) and Pierre Fabre today announced a strategic part...
I-Mab Announces Upcoming Participation at July Conference
SHANGHAI and GAITHERSBURG, Md., June 29, 2021 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical stage biopharmaceutical company committed to the discovery, development and commercialization of novel biologics, today announced its participation in the following conference in July. D...
Medical Affairs Teams are Increasingly Turning to Patient-centric Analytics to Improve Clinical Outcomes
SANTA CLARA, Calif., June 29, 2021 /PRNewswire/ -- The shift to value-based care relies heavily on evidence-based medicine, and pharmaceutical companies play an important role in scientific communication across the healthcare ecosystem. Medical affairs experts interact with healthcare professiona...
Seegene unveils "MOBILE STATION," for on-site routine testing at public-use facilities and communities
* Seegene unveils "MOBILE STATION" at the Medlab Middle East 2021 * Mobile laboratory designed for mass testing at schools, airports, or communities, delivering test results within 3h 30min with the maximum testing capacity of 7,500 a day * The biotechnology firm to sign an MOU with G42 Heal...
Lianhua Qingwen Granted Market Access as Medicine in Ukraine: Yiling Pharmaceutical
SHIJIAZHUANG, China, June 29, 2021 /PRNewswire/ -- Yiling Pharmaceutical disclosed in a statement on Monday that it had received the medicine registration document for Lianhua Qingwen Capsules from the Ministry of Health ofUkraine. As the leading product of Yiling Pharmaceutical, Lianhua Qingwen...
First in China! Neurophth announced the first patient has been dosed in G.O.L.D. clinical trial for the gene therapy treatment of LHON
* Novel and one-time intravitreal injection of ND4 gene therapy for the treatment of LHON potentially restored vision * Phase I/II/III clinical trial expected to enroll patients with LHON due to ND4 mutation WUHAN, China and SAN DIEGO, June 28, 2021 /PRNewswire/ -- Neurophth Biotechnology Ltd...
ILIAS Biologics Inc. demonstrated the therapeutic effect of anti-inflammatory exosomes for ischemia-reperfusion-induced acute kidney injury
DAEJEON, South Korea, June 28, 2021 /PRNewswire/ -- Anti-inflammatory exosomes could have a place in treating acute kidney injury (AKI) caused by ischemia repercussion injury (IRI), according to a study done by ILIAS Biologics, Inc. in collaboration withYonsei University of Seoul, South Korea. ...
RedHill Biopharma's Opaganib Inhibits COVID-19 Variants in Preclinical Study
Opaganib strongly inhibits Beta (South African) and Gamma (Brazilian) COVID-19 variants, further supporting its antiviral activity Opaganib's unique, orally-administered, host-targeted, dual antiviral and anti-inflammatory approach to combatting COVID-19 is also expected to maintain effect agai...
Week's Top Stories
Most Reposted
AVPN's AI Opportunity Fund Expands Regional Efforts to Build AI Skilling Infrastructure for a Future-Ready Workforce Across Asia-Pacific
[Picked up by 301 media titles]
2026-01-21 12:39Government of Telangana and Blaize Sign MoU at Davos to Launch Telangana AI Innovation Hub and Advance Applied AI Initiatives
[Picked up by 296 media titles]
2026-01-22 15:16Infobip strengthens leadership to drive growth and innovation
[Picked up by 281 media titles]
2026-01-16 11:00Klook's study of 374,000 reviews finds that lesser-known cities create deeper emotional connection with travelers
[Picked up by 279 media titles]
2026-01-20 15:40Novo Holdings Invests in Surya Hospitals to Strengthen Access to High-Quality Women's and Children's Healthcare in India
[Picked up by 276 media titles]
2026-01-20 09:00