Pharmaceuticals
European Commission Approves mAbxience's Bevacizumab For The Treatment Of Certain Types Of Cancer
mAbxience, a global fully-fledged biotech company, with over a decade of experience in the development, manufacture and commercialization of biopharmaceuticals, is expanding patient access to biological treatments throughoutEurope. MADRID, March 31, 2021 /PRNewswire/ -- On 31 March 2021, the Eur...
Ajinomoto Bio-Pharma Services Expands Manufacturing Agreement with AstraZeneca to Include Drug Product Manufacturing
WETTEREN, Belgium, March 31, 2021 /PRNewswire/ -- Ajinomoto Bio-Pharma Services ("Aji Bio-Pharma"), a leading provider of biopharmaceutical contract development and manufacturing services, is pleased to announce a manufacturing agreement expansion with AstraZeneca to include drug product manufact...
AffaMed Therapeutics Announces Completion of over US$170 Million Series B Financing Led by Lake Bleu Capital to Further Development of Ophthalmic and Neuroscience Pipeline
SHANGHAI, March 30, 2021 /PRNewswire/ -- AffaMed Therapeutics ("AffaMed"), a global clinical-stage biopharmaceutical company dedicated to addressing critical unmet patient need in ophthalmic, neurological and psychiatric disorders, today announced the completion of over US$170 million in Series B...
GemVax's highly promising Phase II Alzheimer's disease clinical trial results targeting telomerase published in prestigious Alzheimer's Research & Therapy' journal
SEOUL, South Korea, March 30, 2021 /PRNewswire/ -- GemVax & KAEL Co., Ltd. (Korea: 082270)("GemVax") has announced that a paper on GV1001, a novel Alzheimer's treatment based on telomerase modification, was published onMarch 26 th in the SCI-grade international journal 'Alzheimer's Research & Ther...
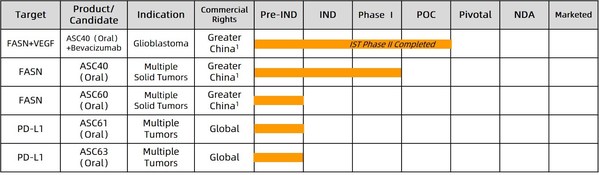
Ascletis Announces Investment Escalation in R&D of Cancer Lipid Metabolism and Oral Checkpoint Inhibitors
* Proposed Initiation of a Pivotal Phase II Trial of ASC40 in Combination with Bevacizumab in Chinese Patients with First Relapse of High-grade Astrocytoma * In-house discovered oral PD-L1 inhibitors demonstrated favorable anti-tumor activities in the animal model compared to a marketed anti-...
Novel Phenomics NASH In Vitro Assay Presented by InSphero AG and PharmaNest Inc at 2021 Society of Toxicology Meeting
Introduction of key technology to accelerate NASH drug discovery by combining 3D disease models and automated AI-based fibrosis quantification PRINCETON, New Jersey, March 30, 2021 /PRNewswire/ -- During the 2021 Society of Toxicology (SOT) Annual Meeting, researchers from Swiss-based InSphero AG...
China Biologic Reports Financial Results for the Fourth Quarter and Fiscal Year 2020
BEIJING, March 29, 2021 /PRNewswire/ -- China Biologic Products Holdings, Inc. (NASDAQ: CBPO, "China Biologic" or the "Company"), a leading fully integrated plasma-based biopharmaceutical company inChina, today announced its financial results for the fourth quarter and fiscal year of 2020. Fourt...
Amplicore, Inc. Announces Closing of a Successful $4M Seed Funding Round
CINCINNATI, March 30, 2021 /PRNewswire/ -- Amplicore Inc., an early-stage biopharmaceutical company currently developing locally delivered and minimally invasive therapeutic solutions for musculoskeletal diseases, has successfully closed its Series Seed round of funding at$4 million. Investments ...
Fosun Pharma Announces 2020 Annual Results
Multiple Innovative Products Launched with Significant Improvement in the Capability of Global Commercialization Net Profit Attributable to Shareholders After Extraordinary G/L Increases 21.65% YOY toRMB2,718 Million SHANGHAI, March 29, 2021 /PRNewswire/ -- Shanghai Fosun Pharmaceutical (...
Tigermed Reports 2020 Annual Results With Solid Growth
HANGZHOU, China, March 29, 2021 /PRNewswire/ -- Hangzhou Tigermed Consulting Co., Ltd. (stock code: 300347.SZ / 3347.HK), a leading provider of innovative clinical research solutions for biopharmaceutical and medical device industry, reports its annual results for the year endedDecember 31, 2020 ...
Kazia Licenses Rights to Paxalisib in Greater China to Simcere, a Leading Chinese Pharmaceutical Company
SYDNEY, March 29, 2021 /PRNewswire/ -- Kazia Therapeutics Limited (ASX: KZA; NASDAQ: KZIA), an oncology-focused drug development company, is pleased to announce that it has entered into a licensing agreement with Simcere Pharmaceutical Group Ltd (Simcere) (HKSE: 2096) to develop and commercialise...
Johnson & Johnson Announces Advance Purchase Agreement with the African Vaccine Acquisition Trust for the Company's COVID-19 Vaccine Candidate
NEW BRUNSWICK, N.J., March 29, 2021 /PRNewswire/ -- Janssen Pharmaceutica NV, one of the Janssen Pharmaceutical Companies of Johnson & Johnson (NYSE: JNJ) (the Company), has entered into an agreement with the African Vaccine Acquisition Task Team (AVATT) to make available up to 220 million dos...
Jacobio Pharma Announces CNY486 Million Revenue for 2020 Annual Results
BEIJING and BOSTON, March 29, 2021 /PRNewswire/ -- Jacobio Pharmaceuticals (1167.HK) announced its first annual results after itsHong Kong listing. By the end ofDecember 31, 2020, its annual revenue was 486 million yuan and R&D expenses exceeded230 million yuan and increased 66% compared to 2019....
Henlius Plans to File the NDA of Novel anti-PD-1 mAb HLX10 for the Treatment of MSI-H Solid Tumours, the Phase 2 Clinical Trial has Met the Primary Endpoint
SHANGHAI, March 29, 2021 /PRNewswire/ -- Shanghai Henlius Biotech, Inc. (2696.HK) announced that the Phase 2 study of its innovative PD-1 inhibitor HLX10 in patients with unresectable or metastatic microsatellite instability-high or mismatch repair-deficient (MSI-H/dMMR) solid tumors that fail t...
Making Most out of Launched Products Fueling Diversified Innovation through Integrated Platform
SHANGHAI, March 28, 2021 /PRNewswire/ -- Henlius (2696.HK) announced the financial results for the year ended31 December 2020. 2020 marked a meaningful year for Henlius as the Company gathered the pace in commercialization and continued the momentum for innovation. The Company delivered a total r...
Foresee Pharmaceuticals Announces Dosing of First Patient in Phase 2/3 Clinical Trial of FP-025 for Treatment of COVID-19 Associated ARDS
TAIPEIMarch 27, 2021 /PRNewswire/ -- Foresee Pharmaceuticals Co., Ltd. (6576.TWO) ("Foresee"), today announced the initiation of patient dosing in the Phase 2/3 clinical trial of FP-025, its highly selective oral MMP-12 inhibitor, in adult patients with severe to critical COVID-19 with associated...
China Pharma Holdings, Inc. Reports Fiscal Year 2020 Financial Results
HAIKOU CITY, China, March 26, 2021 /PRNewswire/ -- China Pharma Holdings, Inc. (NYSE American: CPHI) ("China Pharma," the "Company" or "We"), an NYSE American-listed corporation with a fully-integrated specialty pharmaceuticals subsidiary based inChina, today announced financial results for the ...
Genscript Biotech Reports Full Year 2020 Financial Results
Highlights: * Revenue of the Group for the year ended December 31, 2020 was approximately US$390.8 million, representing an increase of 42.9% as compared with approximatelyUS$273.4 million for the year ended December 31, 2019 * Gross profit of the Group for the year ended December 31, 2...
Modern Chinese Medicine Announces Year 2020 Annual Results
Solid growth with focus to broaden the distribution network and raise R&D efforts PERFORMANCE HIGHLIGHTS * The Group posted a consolidated revenue of approximately RMB308.7 million for the year ended31 December 2020, representing an increase of approximately 41.1%. * For the year ended 31 D...
Saxenda® recommended for approval by European Medicines Agency committee for the treatment of obesity in adolescents aged 12-17 years
BAGSVÆRD, Denmark, March 26, 2021 /PRNewswire/ -- Novo Nordisk today announced that the Committee for Medicinal Products for Human Use (CHMP) under the European Medicines Agency (EMA) has recommended that the use of Saxenda® is expanded for the treatment of obesity in adolescents aged 12–17 years...
Week's Top Stories
Most Reposted
Visa Supports Chinese Cardholders to Add Cards to Apple Pay for a More Convenient and Secure Payment Experience
[Picked up by 309 media titles]
2026-01-15 13:00Ready Server Announces Strategic Expansion into Japan with AT TOKYO Data Centre
[Picked up by 301 media titles]
2026-01-13 13:16JIOS Aerogel® Secures Hyundai Contract Through New Licensing Model
[Picked up by 299 media titles]
2026-01-15 10:00Infobip strengthens leadership to drive growth and innovation
[Picked up by 280 media titles]
2026-01-16 11:00Docquity Launches Engage™, an AI and Insights-Powered HCP Intelligence Engine for the Life Sciences Industry
[Picked up by 278 media titles]
2026-01-15 13:00