Pharmaceuticals
PharmAbcine presents the non-clinical data of PMC-309 at AACR 2021
DAEJEON, South Korea, April 12, 2021 /PRNewswire/ -- PharmAbcine Inc. (KOSDAQ: 208340ks), a clinical-stage biotech company focusing on the development of antibody therapeutics, announced today that the company presented an e-poster featuring the non-clinical data of PMC-309 at American Associatio...
Yiling Pharmaceutical doubled its performance through innovation in 2020
SHIJIAZHUANG, China, April 12, 2021 /PRNewswire/ -- Yiling Pharmaceutical (002603.SZ) released its 2020 annual report on Thursday. It achieved an operating revenue ofRMB 8.782 billion in 2020, with a year-on-year growth of 50.76%; and net profit attributed to equity holders ofRMB 1.219 billion, w...
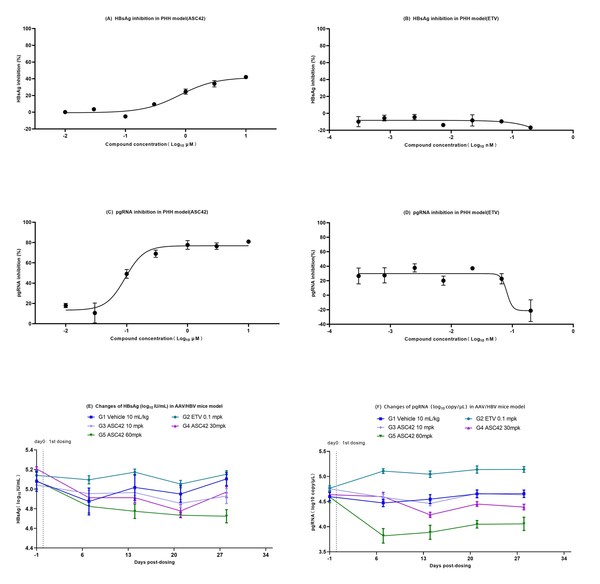
Ascletis Announced Four Clinical and Preclinical Study Abstracts of NASH and HBV Accepted as Poster Presentations by the International Liver Congress™ 2021
HANGZHOU, China and SHAOXING, China, April 12, 2021 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX:1672) announces today that four clinical and preclinical study abstracts of NASH and HBV have been accepted by the International Liver Congress™ 2021 as poster presentations. The summary of the four abs...
Antengene Announces First Patient Dosed in Phase II Trial of ATG-008 (Onatasertib) in Patients with Advanced Solid Tumors with Specific Genetic Alterations
SHANGHAI and HONG KONG, April 12, 2021 /PRNewswire/ -- Antengene Corporation Limited ("Antengene", SEHK: 6996.HK), a leading innovative biopharmaceutical company dedicated to discovering, developing and commercializing global first-in-class and/or best-in-class therapeutics in hematology and onco...
Gracell Biotechnologies Reports Long-term Follow-up Data on TruUCAR-enabled GC027 in Relapsed/Refractory T-ALL at the AACR 2021 Annual Meeting
SUZHOU and SHANGHAI, China, April 10, 2021 /PRNewswire/ -- Gracell Biotechnologies Inc. (NASDAQ: GRCL) ("Gracell"), a global clinical-stage biopharmaceutical company dedicated to developing highly efficacious and affordable cell therapies for the treatment of cancer, today presented updated long...
RedHill Biopharma's Phase 2/3 COVID-19 Study of Opaganib Passes Fourth DSMB Review with Unanimous Recommendation to Continue
Independent Data Safety Monitoring Board unanimously recommends continuation of the global Phase 2/3 study of orally-administered opaganib in severe COVID-19 pneumonia based on review of unblinded safety data from the first 255 treated patients The 464-patient global Phase 2/3 COVID-19 study is ...
Everest Medicines Announces that Licensing Partner Gilead Sciences, Inc. has Received Full US FDA Approval of Trodelvy® for the Treatment of Previously Treated Unresectable Locally Advanced or Metastatic Triple-Negative Breast Cancer
SHANGHAI, April 9, 2021 /PRNewswire/ -- Everest Medicines
CanSinoBIO Announces Approval for its Single-Dose COVID-19 Vaccine Convidecia™ in Chile
* Marks vaccine's first approval in South America, following Mexico, Pakistan, China and Hungary * Safe, stable storage and transportation between 2°C and 8°C, accessible by under-developed regions * 95.47% effective overall in preventing severe COVID-19 diseases 14 days after vaccination TI...
ImmVira Announces Preclinical and Clinical Data to Be Presented at the 2021 ASCO and AACR Annual Meeting
SHENZHEN, China, April 8, 2021 /PRNewswire/ -- ImmVira today announces that the company will be presenting its first innovative product, MVR-T3011, at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting onJune 4-8 2021. Results from the first Phase 1 study of MVR-T3011 as an intr...
EyeYon Medical Announces Innovative Medical Device Designation Granted For EndoArt® In China
TEL-AVIV, Israel, April 8, 2021 /PRNewswire/ -- EyeYon Medical, an Israeli start-up company developing a variety of ophthalmic products for vision-threatening conditions, announces today that EndoArt® was granted the Innovative Device Status byChina's Center for Medical Device Evaluation, the ar...
Inmagene R&D Team expands with two new senior members joining the Company
SAN DIEGO and SHANGHAI and HANGZHOU, China, April 7, 2021 /PRNewswire/ -- Inmagene Biopharmaceuticals ("Inmagene") today announced that Dr.Anbo Xiang and Dr.Qian Xu joined the company. As CMO (China) and Senior VP, Operations, Dr. Xiang will be responsible for clinical development, operations, an...
WuXi Biologics Successfully Completed Pre-License Inspection and Routine GMP Inspection by U.S. FDA
WUXI, China, April 7, 2021 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a global company with leading open-access biologics technology platforms, today announced that it has successfully completed both a Pre-License Inspection (PLI) and a routine GMP inspection by the U.S. FDA (FDA). T...
Bridge Biotherapeutics Announces Initiation of Phase 1/2 Clinical Trial of BBT-176 in EGFR-Mutant NSCLC with C797S
SEONGNAM, South Korea, April 6, 2021 /PRNewswire/ -- Bridge Biotherapeutics Inc.(288330 KQ), a clinical-stage biotech company headquartered in Seongnam, Republic of Korea, announced that the company has initiated the Phase 1/2 clinical trial assessing safety, tolerability, and anti-tumor activity...
Shanghai Genechem Co., Ltd. (Genechem) Announces Global Collaboration on Bispecific Antibodies
SHANGHAI, April 6,, 2021 /PRNewswire/ -- Shanghai Genechem Co., Ltd. (Genechem), a discovery company dedicated to novel drug target discovery and development of novel therapeutics, today announced the execution of a global collaboration with I-Mab (Nasdaq: IMAB), a Nasdaq-listed global bioph...
Gan & Lee receives EMA orphan drug designation for Phase I drug candidate GLR2007 for the treatment of glioma
BEIJING, and BRIDGEWATER, N.J., April 6, 2021 /PRNewswire/ -- Gan & Lee Pharmaceuticals Co., Ltd. (hereinafter referred to as Gan & Lee, stock code: 603087.SH), a global biopharmaceutical company, today announced that the European Medicine Agency (EMA) Committee for Orphan Medicinal Products ...
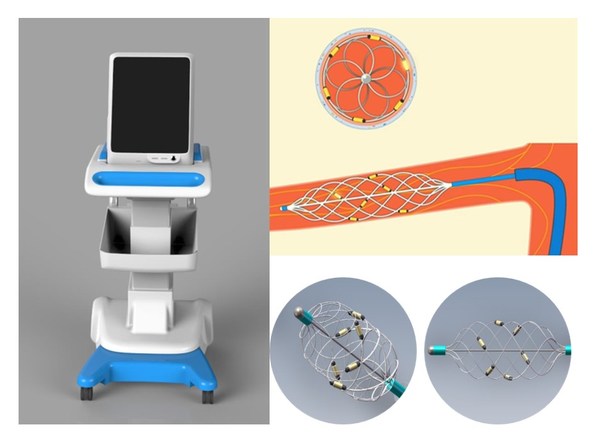
Brattea, a Leading Renal Denervation Company in China, Completes over $US20 Million Series B+ Financing, led by Kuanping Capital
SHANGHAI, April 6, 2021 /PRNewswire/ -- Brattea, a leading renal denervation (RDN) medical technology company inChina, announced today its successful completion of over$US20 million Series B+ financing led by healthcare private equity fund Kuanping Capital. New investors include Hengxu Capital, P...
MitoImmune Received FDA Clearance of IND Application for MIT-001, a Novel Anti-Inflammatory/Anti-Necrotic Therapy for Oral Mucositis in CCRT patients with Head and Neck Cancer
- MitoImmune
Antengene Announces NMPA Approval of IND Application for ATG-019 in Patients with Advanced Solid Tumors or Non-Hodgkin's Lymphoma
SHANGHAI and HONG KONG, April 6, 2021 /PRNewswire/ -- Antengene Corporation Limited ("Antengene", SEHK: 6996.HK), a leading innovative biopharmaceutical company dedicated to discovering, developing and commercializing global first-in-class and/or best-in class therapeutics in hematology and oncol...
Novavax Initiates COVID-19 Vaccine Clinical Trial Crossover
* Crossover allows participants to continue in trials and remain blinded * Ensures that all trial participants receive active vaccine * South Africa and UK crossover arms initiated; US/Mexico PREVENT-19 crossover planned GAITHERSBURG, Md., April 6, 2021 /PRNewswire/ -- Novavax, Inc. (Nasdaq:...
AI-Based Imaging Analysis Tools and Cloud-Based Deployments to Bolster the Global Digital Pathology Market
SANTA CLARA, Calif., April 5, 2021 /PRNewswire/ -- Frost & Sullivan's recent analysis, AI-Based Image Analysis Tools and Cloud-Based Deployment to Expand the Global Digital Pathology Market, finds that this market is estimated to hit $825.9 million by 2025 from $513.3 million in 2019 at a compoun...
Week's Top Stories
Most Reposted
Visa Supports Chinese Cardholders to Add Cards to Apple Pay for a More Convenient and Secure Payment Experience
[Picked up by 311 media titles]
2026-01-15 13:00Colebrook Bosson Saunders Launches Lana: An Adaptable Laptop Stand Engineered for Hybrid Work
[Picked up by 305 media titles]
2026-01-12 16:16Ready Server Announces Strategic Expansion into Japan with AT TOKYO Data Centre
[Picked up by 301 media titles]
2026-01-13 13:16CAYIN AI: Revolutionizing Creativity and Global Communication
[Picked up by 300 media titles]
2026-01-12 16:34JIOS Aerogel® Secures Hyundai Contract Through New Licensing Model
[Picked up by 299 media titles]
2026-01-15 10:00