Health
AGC Biologics Announces the Expansion of Their Cell and Gene Therapy Facility in Milan, Italy
SEATTLE, March 3, 2021 /PRNewswire/ -- AGC Biologics, a leading global Biopharmaceutical Contract Development and Manufacturing Organization (CDMO), announced plans for the expansion of their Cell and Gene Therapy Center of Excellence inMilan, to increase capacities and implement viral vector su...
EZZ Life Science Holdings Limited to list on the ASX today following oversubscribed A$6m IPO
SYDNEY, March 3, 2021 /PRNewswire/ -- Australian beauty and wellness product company, EZZ Life Science Holdings Limited is set to list on the Australian Securities Exchange (ASX) today after raisingA$6 million through its successful and oversubscribed Initial Public Offer. EZZ shares are due to ...
VUNO presents its state-of-the-art AI medical imaging technology at ECR 2021
SEOUL, South Korea, March 2, 2021 /PRNewswire/ -- VUNO Inc., South Korean AI medical imaging company, will showcase its complete deep learning-based imaging offerings two years in a row in its virtual booth at the 2021 European Congress of Radiology (ECR), one of the biggest annual events, featur...
Cipla Gulf Expands Partnership with Alvotech for Commercialization of Biosimilars in Australia and New Zealand
MUMBAI, India, March 2, 2021 /PRNewswire/ -- Cipla Limited (BSE: 500087) (NSE: CIPLA EQ) referred to as "Cipla" today announced that its subsidiary, Cipla Gulf FZ LCC ("Cipla Gulf') is expanding its partnership with Alvotech for the marketing and distribution of four biosimilar medicines inAustra...
Sunquest Lauded by Frost & Sullivan for Driving Efficiency and Compliance with Its Differentiated Molecular Laboratory Information Management System
Sunquest provides various interoperable products and services that work with each other to improve lab efficiency and results accuracy LONDON, March 2, 2021 /PRNewswire/ -- Based on its recent analysis of the North American laboratory information management systems (LIMS) market for molecular an...
Ambu Acclaimed by Frost & Sullivan for Its Groundbreaking Single-use Flexible Endoscopes
Ambu's customer-focused innovations and use of advanced technologies help the company address market needs in a wide range of applications SANTA CLARA, Calif., March 2, 2021 /PRNewswire/ -- Based on its recent analysis of the global flexible single-use endoscope market for advanced visualization...
Cloud-based Enterprise Imaging to Help Expedite Healthcare Providers' Journey of Enabling Better Care
SANTA CLARA, Calif., March 2, 2021 /PRNewswire/ -- Responding to the shift toward value-based care, provider M&A and consolidation, data expansion, and analytics innovations, healthcare providers are accelerating their digital transformation for greater efficiency, automation and access across cl...
Global Cord Blood Corporation Announces Receipt of Unsolicited Non-Binding Acquisition Proposal
HONG KONG, March 2, 2021 /PRNewswire/ -- Global Cord Blood Corporation (NYSE: CO) ("GCBC" or the "Company"),China's leading provider of cord blood collection, laboratory testing, hematopoietic stem cell processing and stem cell storage services, today announced that its board of directors (the "...
Merck and GPHL Collaborate on Business Innovation and Development in the Greater Bay Area
GUANGZHOU, China, March 2, 2021 /PRNewswire/ -- Merck, a leading science and technology company, today signed a Memorandum of Understanding (MOU) to begin strategic collaboration with Guangzhou Pharmaceutical Holdings Limited (GPHL), China's leading pharmaceutical company. The collaboration aim...
Cipla receives final approval for generic version of GlaxoSmithKline's IMITREX® (Sumatriptan Nasal Spray, 20 mg)
MUMBAI, India, March 2, 2021 /PRNewswire/ -- Cipla Limited (BSE: 500087) (NSE: CIPLA EQ) (hereinafter referred to as "Cipla") today announced that it has received final approval for its Abbreviated New Drug Application (ANDA) for Sumatriptan Nasal Spray, 20 mg from the United States Food and Drug...
Oscotec and Beactica Therapeutics announce license and collaboration agreement to develop new cancer drug
STOCKHOLM, March 2, 2021 /PRNewswire/ -- Oscotec Inc. (039200: KOSDAQ), the Korean drug development company, and Beactica Therapeutics AB, the Swedish drug discovery company, today announced a new research development and licensing agreement. Oscotec and Beactica will initially jointly collaborat...
CMAB Biopharma Congratulates QureBio on FDA Clearance of IND Application for Claudin18.2/PD-L1 Bispecific Antibody
SUZHOU, China, March 2, 2021 /PRNewswire/ -- Recently, CMAB Biopharma (Suzhou) Inc's ("CMAB") partner QureBio Ltd ("QureBio") has announced its innovative drug Q-1802 received United States Food and Drug Administration (FDA) clearance for an Investigational New Drug (IND) application. This applic...
Kazia Licenses Cantrixil, a Clinical-stage, First-in-class Ovarian Cancer Drug Candidate, to Oasmia Pharmacetical AB
SYDNEY, March 2, 2021 /PRNewswire/ -- Kazia Therapeutics Limited (ASX: KZA; NASDAQ: KZIA), an Australian oncology-focused biotechnology company, is pleased to announce that it has entered into an exclusive worldwide license agreement with Oasmia Pharmaceutical AB (STO: OASM), an innovation-focuse...
INOVIO Announces Positive Results from REVEAL 1, a Phase 3 Pivotal Trial Evaluating VGX-3100, its DNA-based HPV Immunotherapy for the Treatment of High-grade Precancerous Cervical Dysplasia Caused by HPV-16 and/or HPV-18
Trial achieved primary and secondary efficacy endpoints among all evaluable subjects in the Phase 3 multi-center, randomized, double-blind, placebo-controlled trial VGX-3100 is the first DNA medicine to achieve efficacy endpoints in a Phase 3 clinical trial INOVIO also continues to partner with...
New NCCN Guidelines for Histiocytosis Clarify Best Practices for Recently-Defined Cancers
New clinical practice guidelines from National Comprehensive Cancer Network focus on workup and management for three main adult histiocytic disorders. PLYMOUTH MEETING, Pa., March 1, 2021 /PRNewswire/ -- The National Comprehensive Cancer Network® (NCCN®)—an alliance of leading cancer centers —tod...
China Biologic Announces Shareholders' Approval of Merger Agreement
BEIJING, March 1, 2021 /PRNewswire/ -- China Biologic Products Holdings, Inc. (NASDAQ: CBPO, "China Biologic" or the "Company"), a leading fully integrated plasma-based biopharmaceutical company inChina, today announced that, at an extraordinary general meeting (the "EGM") held today, the Company...
BioVaxys Expanding Technology Platform To Address Emerging SARS-CoV-2 Variants
BVX-0320 and Covid-T to have capability to address UK, Brazilian and South African Virus Variants VANCOUVER, B.C., March 1, 2021 /PRNewswire/ -- BioVaxys Technology Corp. (CSE: BIOV) (FRA: 5LB) (OTC: LMNGF)("BioVaxys"), the world leader in haptenized protein vaccines for antiviral and cancer app...
Frost & Sullivan Reveals Virtual Care's Enormous Potential in the United States
The US virtual care market is expected to witness more than a seven-and-a-half-fold growth by 2025, with a CAGR of 40.4% SANTA CLARA, Calif., March 1, 2021 /PRNewswire/ -- Frost & Sullivan's recent analysis, COVID-19 Pandemic Ignites Enthusiasm for Virtual Care, finds that virtual care is the ne...
Medilink Therapeutics Raises $50 Million in Series A Financing To Accelerate Next-Generation Conjugated Drugs Research & Development
SUZHOU, China, March 1, 2021 /PRNewswire/ -- Suzhou Medilink Therapeutics Ltd. (Medilink), a global biotech company focusing on next-generation antibody-drug conjugate Research & development, announced the closing of a$50 million series A financing, to accelerate its innovative pipeline developme...
CARsgen Therapeutics Receives Orphan Medicinal Product Designation from the European Medicines Agency for CT041 CLDN18.2 CAR T Cells for the Treatment of Gastric Cancers
SHANGHAI, March 1, 2021 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited, a clinical-stage biopharmaceutical company, today announced that the European Commission (EC) has granted orphan designation for CT041, CARsgen's first-in-class Claudin 18.2 (CLDN18.2) targeted CAR-T product candidate ...
Week's Top Stories
Most Reposted
Going Global: DCITS Embarks on International Expansion at Singapore Fintech Festival
[Picked up by 313 media titles]
2024-11-12 09:00DKSH Healthcare and Euris Unveil CRM & MCE Platform "ConnectPlus" to Revolutionize APAC Healthcare Distribution
[Picked up by 292 media titles]
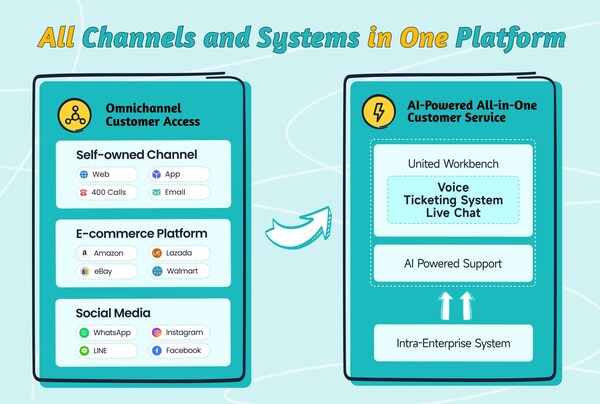
2024-11-13 09:00Sobot Introduces its All-in-One Solution at GITEX Global 2024
[Picked up by 291 media titles]
2024-11-12 11:00Ubiqconn Technology to Showcase Latest Marine Solutions at the 2024 International WorkBoat Show in New Orleans
[Picked up by 289 media titles]
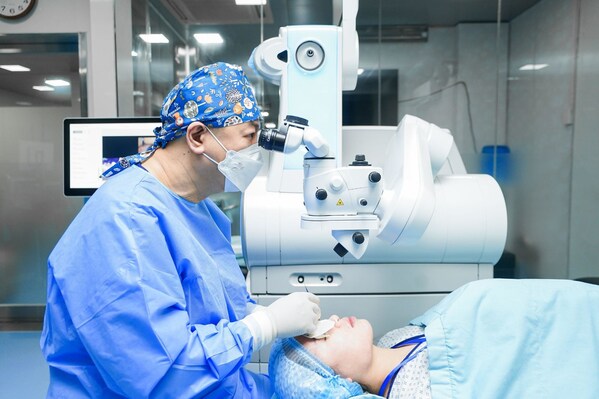
2024-11-11 21:00DND International Eye Hospital: Pioneering SMILE Pro Surgery & Leading the Trend of Refractive Surgery Tourism in Vietnam
[Picked up by 279 media titles]
2024-11-08 20:11