Medical/Pharmaceuticals
Mentice publishes the company's year-end report for the period January-December 2020
STOCKHOLM, Feb. 4, 2021 /PRNewswire/ -- Strong order intake and substantial improvement of the company's order book as well as operating cash flow Fourth quarter (October-December 2020) * Order intake amounted to 73.1 (54.7) MSEK. * Net sales amounted to 45.9 (60.2) MSEK. * Ope...
Vemlidy(r) Demonstrates Continued Efficacy and Improved Safety for Asian Patients with Hepatitis B
SINGAPORE, Feb. 4, 2021 /PRNewswire/ -- Gilead Sciences announced today at the 2021 Asian Pacific Association for the Study of the Liver Conference (APASL), findings from two sub-analyses that demonstrated the continued efficacy and improved safety of Vemlidy® (tenofovir alafenamide 25 mg, TAF) ...
NCCN Gets Personal About Improving Global Cancer Care for World Cancer Day
Nonprofit alliance behind gold standard oncology guidelines shares stories of personal connections to cancer that inspire many within the organization. Global oncology community looks at the impact from COVID-19 on cancer care over the past year. PLYMOUTH MEETING, Pa., Feb. 4, 2021 /PRNewswire/ ...
Edogawa Hospital in Tokyo Installs Japan's First ViewRay MRIdian® Linac
CLEVELAND, Feb. 4, 2021 /PRNewswire/ -- ViewRay, Inc. (Nasdaq: VRAY) announced today that Edogawa Hospital in Tokyo is the first hospital inJapan to treat patients with the MRIdian Linac System. Edogawa Hospital began treating patients in 2018 on the MRIdian cobalt system. The upgrade to MRIdi...
FDA Approves TEPMETKO® as the First and Only Once-daily Oral MET Inhibitor for Patients with Metastatic NSCLC with METex14 Skipping Alterations
Not intended for distribution in the USA, Canada or UK - TEPMETKO is approved for both treatment naïve and previously treated MET ex14 positive NSCLC patients - TEPMETKO demonstrated consistent and durable responses in both treatment naïve and previously treatedMETex14 patients in the VISION stud...
Guardant360 CDx submitted for regulatory approval in Japan
TOKYO, Feb. 3, 2021 /PRNewswire/ -- Guardant Health Japan, an affiliate of Guardant Health Asia,Middle East & Africa (AMEA) has announced that it has submitted its application to the Ministry of Health, Labour and Welfare (MHLW) for regulatory approval of Guardant360 CDx, a liquid biopsy test for...
Innovent and Lilly Jointly Announce the Approval of TYVYT® (sintilimab injection) by China NMPA in Combination with Pemetrexed and Platinum Chemotherapy as First-Line Therapy for Nonsquamous Non-Small Cell Lung Cancer
SAN FRANCISCO and SUZHOU, China, Feb. 3, 2021 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of cancer, metabolic, autoimmune and other major disease...
Ping An Healthcare and Technology Company Limited posts revenue of RMB 6,866 million in 2020
Launches comprehensive strategic upgrade Revenue from online medical services, its core business, grows 82.4% year-on-year HONG KONG, Feb. 3, 2021 /PRNewswire/ -- Ping An Healthcare and Technology Company Limited ("Ping An Good Doctor"; Stock Code: 01833.HK), a leading internet healthcare se...
Elekta's MOSAIQ and Versa HD earn top honors in 2021 Best in KLAS: Software and Services Report
ATLANTA, Feb. 3, 2021 /PRNewswire/ -- Elekta (EKTA-B.ST) today announced that itsMOSAIQ® Oncology Information System (OIS) and Versa HD™ linear accelerator have been named "Best in KLAS" in the 2021 Best in KLAS: Software and Services Report. MOSAIQ was ranked first in the Oncology (Radiation) ca...
Mindray Launches New BeneFusion e Series Infusion Systems, Delivering Efficiency in Every Droplet
SHENZHEN, China, Feb. 2, 2021 /PRNewswire/ -- Mindray (SZSE: 300760), a global leader in the development of innovative healthcare technology, has unveiled its BeneFusion e Series, a revolutionary efficient infusion system for the healthcare industry. Available in three models, eSP, eVP and eDS,...
Kira Pharmaceuticals Announces First Participant Dosed in Phase 1 Clinical Trial for P014, a Bifunctional Biologic Medicine
CAMBRIDGE, Mass. and SUZHOU, JIANGSU, China, Feb. 2, 2021 /PRNewswire/ -- Kira Pharmaceuticals, a global biotechnology company pioneering a new generation of complement-targeted therapies to treat immune-mediated diseases, announced today that the first healthy volunteer has been successfully dos...
Ascentage Pharma Released Preclinical Results of MDM2-p53 Inhibitor APG-115 in an Oral Presentation at WCLC 2020, Demonstrating Therapeutic Potential in STK11-Mutant Non-Small Cell Lung Cancer
SUZHOU, China and ROCKVILLE, Md., Feb. 2, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, announced that the company recently released ...
Globally unique MRI Guarantee: The highest MRI safety with hearing implants from MED-EL
Worry-free, safe magnetic resonance imaging (MRI) with MED-EL cochlear, middle ear or bone conduction implants – now with lifetime guarantee. For immediate access to an MRI examination without surgery, discomfort or hearing downtime. INNSBRUCK, Austria, Feb. 2, 2021 /PRNewswire/ -- The innovative...
Inmagene launched U.S. subsidiary; Dr. Jean-Louis Saillot joined the company.
SAN DIEGO, Calif. and SHANGHAI, Feb. 2, 2021 /PRNewswire/ -- Inmagene Biopharmaceuticals ("Inmagene"), a leading biotech company focused on immunology-related therapeutic areas, today announced the launch of its wholly-owned subsidiary inSan Diego. Jean-Louis Saillot, MD has joined the company a...
CF PharmTech and Chengdu Shangyi Launch the "Home-Based Recovery Program for Discharged Covid-19 Patients" and Announce Today's Global Release of the "R Plus Health" Free App
SUZHOU, China, Feb. 2, 2021 /PRNewswire/ -- CF PharmTech, Inc. is a fully
integrated pharmaceutical company inChina specializing in the treatment of
respiratory diseases. Chengdu Shangyi Information Technology Co., Ltd is a
professional IT service company in the healthcare industry inChina.
MedTech Startup See-Mode Technologies Announces CE Mark and Australian TGA Approval for AI-powered Ultrasound Analysis Software
SINGAPORE and MELBOURNE, Australia, Feb. 2, 2021 /PRNewswire/ -- See-Mode
Technologies
Crafoord Laureate discovered the explanation for mysterious fevers
STOCKHOLM, Feb. 1, 2021 /PRNewswire/ -- Dan Kastner, USA, has described an entirely new group of rare autoinflammatory diseases. His discoveries have brought new knowledge and led to the development of effective treatments. The Royal Swedish Academy of Sciences is awarding him this year's Crafoor...
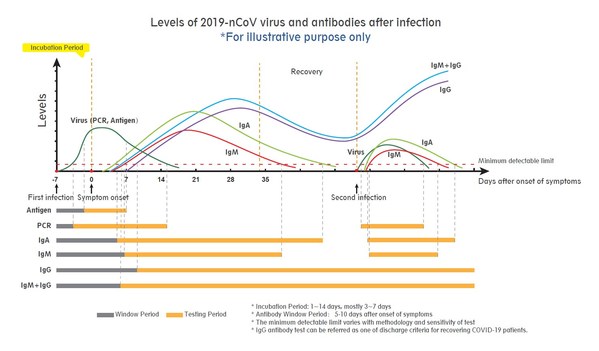
Wondfo - Neutralizing antibody testing, antigen testing and PCR key in battle against COVID-19 in 2021
GUANGZHOU, China, Feb. 1, 2021 /PRNewswire/ -- Wondfo is ramping up neutralizing antibody, antigen and PCR tests to address the evolving demands for COVID-19 testing globally. As countries draw up plans to deal with the new wave of infections amidst the pandemic while vaccination programmes are r...
Assisted Reproduction Unicorn Basecare Medical to IPO in Hong Kong
SUZHOU, China, Feb. 1, 2021 /PRNewswire/ -- Suzhou Basecare Medical Co., Ltd. (Basecare Medical) — set to list on the Hong Kong Stock Exchange onFebruary 8th , 2021— is the newest unicorn in the field of assisted reproduction. The planned IPO comes after a very successful and profitable 2020, favo...
Clover and Dynavax Announce Planned Global Phase 2/3 Efficacy Trial of Adjuvanted COVID-19 Vaccine Candidate
* Clover plans to initiate a global Phase 2/3 efficacy trial of its protein-based S-Trimer COVID-19 vaccine candidate adjuvanted with Dynavax's CpG 1018 plus alumin the first half of 2021 with an interim analysis for vaccine efficacy potentially in the middle of 2021. * The Coalition for Epid...
Week's Top Stories
Most Reposted
Earth Day 2024: Angel Yeast Continues to Tackle Plastic Pollution Challenges With Bio-based Material Solutions
[Picked up by 294 media titles]
2024-04-22 16:00Trina Solar and PetroGreen Partner to Accelerate Philippine Solar Adoption with 117MW Supply Agreement
[Picked up by 291 media titles]
2024-04-22 06:00Revenue Surpasses 50 Billion: BlueFocus Accelerates Towards the AI Native Era
[Picked up by 276 media titles]
2024-04-23 15:43INTAMSYS Becomes 3D Printing Equipment Supplier for the WORLDSKILLS LYON 2024 COMPETITION
[Picked up by 274 media titles]
2024-04-24 17:09Introducing Wacom Movink: The first OLED pen display, and the thinnest and lightest Wacom pen display ever
[Picked up by 268 media titles]
2024-04-24 13:00