Pharmaceuticals
Innovent Presents the Results of the First Phase 3 Study of Mazdutide for Weight Management at the ADA's 84th Scientific Sessions
SAN FRANCISCO and SUZHOU, China, June 25, 2024 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, autoimmune, cardiovascular and metabolic, ...
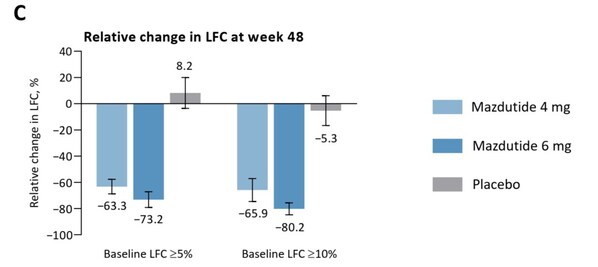
Innovent Announces Mazdutide Demonstrates 80.2% Reduction in Liver Fat Content in Exploratory Analysis of Phase 3 Weight Management GLORY-1 Study at ADA 2024
SAN FRANCISCO and SUZHOU, China, June 25, 2024 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, autoimmune, cardiovascular and metabolic, ...
Juniper Biologics Expands Distribution Rights for Caris Life Sciences' Molecular Profiling in the Middle East and Africa
SINGAPORE, June 24, 2024 /PRNewswire/ -- Singapore-headquartered Juniper Biologics Pte Ltd (Juniper), a leading healthcare and pharmaceuticals company focused on commercialising novel therapies, has been granted distribution rights for Caris Life Sciences (Caris)' solid tumour molecular profiling...
Ascentage Pharma Announces Confidential Submission of Draft Registration Statement for Proposed Initial Public Offering of American Depositary Shares
ROCKVILLE, Md. and SUZHOU, China, June 24, 2024 /PRNewswire/ -- Ascentage Pharma (6855.HK) announced that it has confidentially submitted a draft registration statement on Form F-1 to the U.S. Securities and Exchange Commission (the "SEC") relating to the proposed initial public offering of Amer...
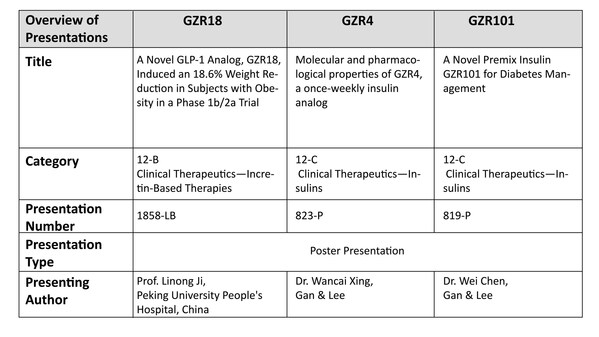
Gan & Lee Pharmaceuticals Announces Significant Progress on New Diabetes and Obesity Treatments at the American Diabetes Association's 84th Scientific Sessions
BEIJING and BRIDGEWATER, N.J., June 23, 2024 /PRNewswire/ -- Gan & Lee Pharmaceuticals (Gan & Lee, Shanghai Stock Exchange: 603087) announced the results of the Phase1b/2a clinical study of the Company's independently developed glucagon-like peptide-1 (GLP-1) receptor agonist, GZR18 Injection, in...
Sciwind Biosciences to Highlight Positive Results for Injectable Ecnoglutide (Phase 3), Oral Ecnoglutide (Phase 1), and Novel Amylin Analogs at the American Diabetes Association (ADA) 84th Annual Conference
* A Phase 1 study of oral ecnoglutide (1871-LB) showed it to be safe and well tolerated and result in pronounced weight loss (up to -6.76% after 6 weeks of dosing). Improved oral bioavailability enables a 15 to 30 mg daily dose of oral ecnoglutide to match or exceed the plasma exposure of weekl...
Biocytogen Awarded U.S. Patent for RenLite® Common Light Chain Mouse Platform
BEIJING, June 21, 2024 /PRNewswire/ -- Biocytogen Pharmaceuticals (Beijing)
Co., Ltd. ("Biocytogen", HKEX: 02315) announced United States Patent and
Trademark Office (USPTO) patent grant for independently developed RenLite®
fully human common light chain mouse platform.
Ascentage Pharma Announces Closing of US$75 Million Equity Investment by Takeda
ROCKVILLE, Md. and SUZHOU, China, June 21, 2024 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancer, chronic hepatitis B (CHB), and age-related diseases, announced today that the agreed equity investment by Takeda has bee...
Clarivate Identifies Seven Innovators in Antibody Drug Conjugates in New Companies to Watch Report
Emerging standouts set to transform drug discovery and development, revolutionize cancer treatment, and capture big pharma interest highlighted in third annual report LONDON, June 19, 2024 /PRNewswire/ -- Clarivate Plc (NYSE:CLVT), a leading global provider of transformative intelligence, has re...
Innovent Reports Oncology Pipeline Updates at Investor Meeting
SAN FRANCISCO and SUZHOU, China, June 19, 2024 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, metabolic, autoimmune, ophthalmology and o...
Menarini Asia-Pacific partners with Pharmacosmos to expand its reach of MonoFer® to Singapore and Malaysia
SINGAPORE, June 19, 2024 /PRNewswire/ -- Menarini Asia-Pacific (Menarini) today announced that it has expanded its partnership with Pharmacosmos A/S (Pharmacosmos), to include exclusive rights to commercialiseMonoFer® (ferric derisomaltose) in Singapore and Malaysia. This builds upon the successf...
Clover Announces Positive Preliminary Phase Ⅰ Results for Bivalent RSV Vaccine Candidate SCB-1019 in Older Adults
-- Bivalent SCB-1019 significantly boosted RSV-A and RSV-B neutralizing antibody titers in older adults up to approximately 7,900 IU/mL (up to 8-fold increase) and approximately 46,700 IU/mL (up to 11-fold increase), respectively -- -- Favorable safety & reactogenicity profile comparable to sali...
Diplomatic Envoys to China Visit Fosun: Appreciating Oriental Lifestyle Aesthetics and Seeking Opportunities for Business Cooperation
SHANGHAI, June 18, 2024 /PRNewswire/ -- Over 40 guests, including 10 ambassadors toChina, 8 consuls general and other senior diplomats from 26 countries, visited Fosun on 14 June. The delegation toured along the Grand Yuyuan. From the intangible cultural heritage of the Old City of Shanghai to t...
Live from EHA 2024 | Ascentage Pharma Releases Updated Data of Lisaftoclax in Patients with R/R MM and AL Amyloidosis Highlighting Marked Improvement in ORR
SUZHOU, China and ROCKVILLE, Md., June 18, 2024 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancer, chronic hepatitis B (CHB), and age-related diseases, announced today that it has released updated data of the Bcl-2 inh...
Live from EHA 2024 | Posters Featuring Results from Three Studies of Olverembatinib, Including Encouraging Data from US Study in CML and Ph+ ALL
SUZHOU, China and ROCKVILLE, Md., June 18, 2024 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancer, chronic hepatitis B (CHB), and age-related diseases, announced today that updated results from three studies of olverem...
Gan & Lee Pharmaceuticals to Present Groundbreaking Data on Three Innovative Products at the American Diabetes Association's 84th Scientific Sessions
* A phase 1b/2a trial evaluated once- and bi-weekly GZR18, a novel GLP-1 analog, in Chinese subjects with obesity/overweight * Pre-clinical studies evaluated the molecular and pharmacological properties of GZR4, an investigational once-weekly insulin analog * Pre-clinical studies evaluated ...
Faculty of Medicine Siriraj Hospital and Dhulikhel Hospital commemorate 15 years of collaborative relationship
BANGKOK, June 17, 2024 /PRNewswire/ -- In 2024, Thai-Nepalese bilateral relations reached a new milestone as the Faculty of Medicine Siriraj Hospital at Mahidol University inThailand and Dhulikhel Hospital at Kathmandu University inNepal celebrated the 15th anniversary of their esteemed partnersh...
Ascentage Pharma Signs Option Agreement with Takeda to Enter into Exclusive Global License for Olverembatinib, a Third-Generation BCR-ABL Tyrosine Kinase Inhibitor (TKI)
* Ascentage Pharma has executed an Exclusive Option to License Global Rights to Olverembatinib worldwide other thanChina and a few other areas. * Ascentage Pharma to receive an option payment of 100 million USD upon closing and be eligible for an option exercise fee and additional potential m...
Innovent Delivers Oral Presentation on Clinical Data of IBI363 (First-in-class PD-1/IL-2α-bias Bispecific Antibody Fusion Protein) in Advanced Non-small Cell Lung Cancer and Other Solid Tumors at the 2024 ESMO Virtual Plenary
SAN FRANCISCO and SUZHOU, China, June 14, 2024 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, autoimmune, metabolic, ophthalmology ...
Ethypharm expands its Mesalazine (Etiasa®) Portfolio in China
SAINT-CLOUD, France, June 13, 2024 /PRNewswire/ -- Ethypharm is pleased to announce the acquisition of a suppository form of Mesalazine by its Chinese affiliate, Shanghai Ethypharm Pharmaceuticals Ltd, from the Chinese Company Jiangsu Anbison Pharmaceutical. Leader in essential medicines for cen...
Week's Top Stories
Most Reposted
Agoda Launches Free Global eSIMs for VIP Diamond Members
[Picked up by 322 media titles]
2026-02-10 14:00Ascentium Acquires Clara, Expanding into the Abu Dhabi Global Market (ADGM) and Strengthening its Middle East Presence
[Picked up by 310 media titles]
2026-02-12 14:00Blackpanda Japan Announces Strategic Partnership with SoftBank to Strengthen Cyber Incident Response in Japan
[Picked up by 299 media titles]
2026-02-10 13:31Rockwell Automation Strengthens Industrial Cybersecurity with New Security Operations Center in Singapore
[Picked up by 299 media titles]
2026-02-09 10:00Carro unveils quirky generative AI ad campaign highlighting its 'Surprisingly Short' AI-enabled car-selling process
[Picked up by 296 media titles]
2026-02-11 11:00