Pharmaceuticals
Innovent Announces the Second Phase 3 Trial of Mazdutide in Chinese Patients with Type 2 Diabetes Met Study Endpoints, and Plans to Submit NDA of Mazdutide to the NMPA
SAN FRANCISCO and SUZHOU, China, July 22, 2024 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, autoimmune, cardiovascular and metabolic, ...
Rona Therapeutics Raises $35 Million Series A+ Financing to Advance Innovative Metabolic siRNA Pipeline in Clinic and Next Generation RNA Platforms
* Financing led by LongRiver Investments with participation by new and existing global investment funds * Rona will use proceeds to progress leading metabolic siRNA pipeline programs into global development and expand extra-hepatic delivery platform in CNS and beyond SHANGHAI, July 19, 2024 /...
DX&VX is on Track and Accelerating the Development of Oral Obesity Treatment
* Confirmation of effects compared to global late-stage clinical trials * Accelerating the development of obesity treatment through early licensing out, global joint clinical trials, research funding investment, etc. SEOUL, South Korea, July 18, 2024 /PRNewswire/ -- DX&VX, which is gaining att...
Pfizer's First Dual Indication Vaccine for Respiratory Syncytial Virus (RSV) is Available This Month in Hong Kong
* The first and only bivalent, single-dose vaccine that fights against both RSV A and RSV B has been approved by health authorities inHong Kong and Macau earlier, and is available inHong Kong this month (July 2024) * Helps fight against RSV in older adults aged 60 and above and infants (throu...
Everest Medicines Announces Official Acceptance Granted on NEFECON® Supplementary Application by China National Medical Products Administration
SHANGHAI, July 18, 2024 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK, "Everest", or the "Company"), a biopharmaceutical company focused on the discovery, clinical development, manufacturing and commercialization of innovative therapeutics, today announced thatChina's National Medical Products ...
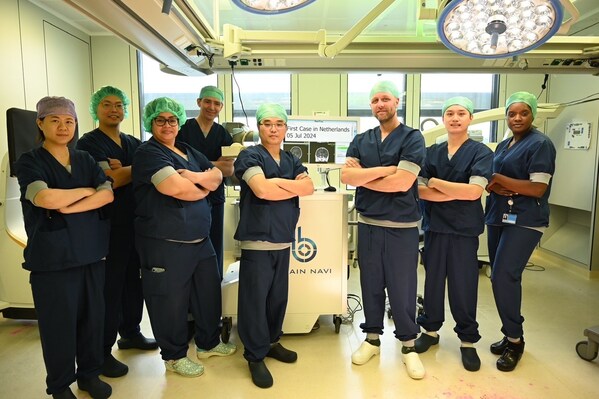
First procedure in Netherlands with NaoTrac surgical navigation robot is performed at Erasmus MC Rotterdam
HSINCHU, July 17, 2024 /PRNewswire/ -- Brain Navi announces the completion of its first case inNetherlands, which was successfully and safely performed at Erasmus MC inRotterdam, Netherlands. Brain biopsy helps doctors decide the best treatment for the patient after taking a tissue sample from t...
CordenPharma Invests €900m in Transformational Peptide Platform Expansion in the USA & Europe
* CordenPharma is making a record investment of ~€900m over the next 3 years in expanding its peptide platform, both at itsColorado, US site and in Europe. The expansions, covering both existing facilities and new constructions, will meet the pharmaceutical industry's most stringent quality and...
SPI Pharma, Inc. and Inimmune, Corp. Partner to Develop and Commercialize Innovative Vaccine Adjuvant Systems
WILMINGTON, Del., July 16, 2024 /PRNewswire/ -- SPI Pharma, Inc. a global leader in biopharmaceutical excipient and adjuvant systems, and Inimmune, Corp., a pioneering biotechnology company specializing in the discovery and development of innate immune modulators, announce an agreement in princip...
Seegene Showcases HPV Diagnostic Kits and PCR Technology at AOGIN 2024
* Showcases Allplex™ HPV products designed to test for 14 high-risk HPV genotypes * Advocates HPV testing for early diagnosis and prevention of cervical cancer * Aims to accelerate the widespread adoption of syndromic PCR testing to create "a world free from all diseases" SEOUL, South Kore...
Biosyngen's BRG01 enters Phase II clinical trial, a first-in-kind autologous EBV-Specific CAR-T Therapy for Solid Tumors on Recurrent/Metastatic Nasopharyngeal Carcinoma
SINGAPORE, July 16, 2024 /PRNewswire/ -- Biosyngen, a leading biotechnology company focused on the development of innovative cell therapies, recently announced that the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) inChina has approved the initiation of a...
CPHI & PMEC Shenzhen 2024: Fostering New Opportunities in Asia and China's Greater Bay Area
SHANGHAI, July 15, 2024 /PRNewswire/ -- CPHI & PMEC China 2024 concluded in June with an exceptional turnout of visitors, setting a new attendance record. Notably, the number of international visitors grew to 11,563, and professionals from the end-to-end pharmaceutical supply chain made meaningfu...
Everest Medicines Announces Positive Topline Results from Maintenance Period of Etrasimod Phase 3 Clinical Trial Conducted in Asia for the Treatment of Moderately-to-Severely Active Ulcerative Colitis
SHANGHAI, July 15, 2024 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK, "Everest", or the "Company"), a biopharmaceutical company focused on the discovery, clinical development, manufacturing and commercialization of innovative therapeutics, today announced positive topline data results of the ...
Basecare Medical (2170. HK) revenue growth exceeds 50% in 2024H1
SUZHOU, China , July 15, 2024 /PRNewswire/ -- Basecare Medical (2170. HK) released an unaudited interim performance forecast onJuly 12th, stating that the company's revenue for the first half of the year was approximatelyRMB 127 million to RMB 138 million, an increase of approximately 49% to 62% ...
Sirnaomics Announces Completion of IND-Enabling Studies of Safety and Efficacy for STP125G with NHP Models, Targeting ApoC3 for Treatment of Cardiovascular Diseases
HONG KONG, GERMANTOWN, Md. and SUZHOU, China, July 12, 2024 /PRNewswire/ -- Sirnaomics Ltd.(the "Company", Stock Code: 2257.HK, together with its subsidiaries, the "Group" or "Sirnaomics"), a leading biopharmaceutical company engaging in discovery and development of advanced RNAi therapeutics, an...
Initiation of cell therapy research and development program for adult T-Cell leukemia / lymphoma (ATLL)
Initiation of cell therapy research and development program for adult T-Cell leukemia / lymphoma (ATLL) TOKYO, July 11, 2024 /PRNewswire/ -- The National Cancer Center (Tokyo, Japan ) and TheUniversity of Pennsylvania (Philadelphia, PA, USA) have licensed patent rights directed to a chimeric...
Telix Welcomes CMS Proposal to Improve Payment for Specialised Diagnostic Radiopharmaceuticals
MELBOURNE, Australia, July 11, 2024 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Telix, the Company) today welcomes proposed changes announced by the Centers for Medicare & Medicaid Services (CMS) for the Hospital Outpatient Prospective Payment System (OPPS) rule to improve payments ...
Eligo Publishes in Nature a Landmark Study That Unlocks Genome Editing of Bacteria in the Gut
PARIS, July 10, 2024 /PRNewswire/ -- For the first time, a team of scientists at Eligo has demonstrated it was possible to genetically modify bacteria with nearly 100% efficiency directly in the gut of animals. This work provides scientists with a novel strategy to better understand how genes fro...
Cambrex to Expand Stability Storage Business with New Facility in Durham, North Carolina
EAST RUTHERFORD, N.J., July 8, 2024 /PRNewswire/ -- Cambrex today announced the expansion of its stability storage business, Q1 Scientific, which offers environmentally-controlled stability storage services to the pharmaceutical, medical device, and life sciences industries. Q1 Scientific will op...
Sirnaomics Announces Interim Results for Successful Completion of the Second Cohort of Phase I Clinical Study of GalNAc-Based RNAi Therapeutic STP122G for Anticoagulant Therapeutics
HONG KONG and GERMANTOWN, Md. and SUZHOU, China, July 8, 2024 /PRNewswire/ -- Sirnaomics Ltd. (the "Company", together with its subsidiaries, the "Group" or " Sirnaomics"; stock code: 2257), a leading biopharmaceutical company engaging in discovery and development of advanced RNAi therapeutics, to...
Olverembatinib Approved for Commercialization in Macau China
ROCKVILLE, Md. and SUZHOU, China, July 8, 2024 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in discovering, developing and commercializing both first- and best-in-class therapies for hematological malignancies, announced today that its novel BCR-ABL1 tyro...
Week's Top Stories
Most Reposted
Agoda Launches Agoda Impact Lab at ASEAN Tourism Forum
[Picked up by 318 media titles]
2026-01-29 15:06Agoda Launches Open-Source API Agent to Simplify MCP Server Integrations
[Picked up by 309 media titles]
2026-01-27 13:00Nokia Strengthens Edge AI Capabilities Through Strategic Collaboration with Blaize on Hybrid Inference Solutions Across Asia Pacific Regions
[Picked up by 294 media titles]
2026-01-27 18:00Singapore Airshow 2026 Milestone Edition: 20 Years of Shaping the Aerospace Landscape as Asia-Pacific Drives Global Growth
[Picked up by 284 media titles]
2026-02-01 19:35Multi-Award Winning Phuket Marriott Resort & Spa, Merlin Beach: Voted Top 3 Best Family Resort
[Picked up by 283 media titles]
2026-01-30 08:00