Pharmaceuticals
Merck Granted U.S. Patents for Foundational CRISPR-Cas9 Technology
- Technology provides more tools to fight difficult-to-treat diseases - Life Science business actively licensing its CRISPR-Cas9 technology for therapeutic and other uses - Committed to ethical use of genome-editing technology DARMSTADT, Germany, May 11, 2020 /PRNewswire/ -- Merck, a leading...
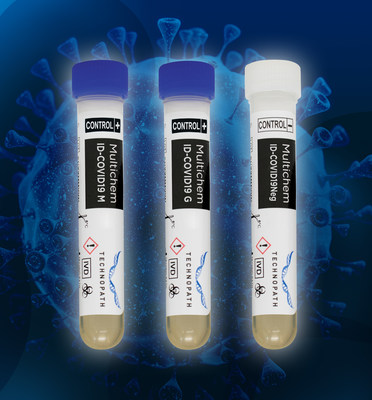
Technopath Clinical Diagnostics Expands Its Portfolio of Third-party Quality Control Solutions for COVID-19 as Clinical Laboratories Globally Ramp Up Antibody Testing
NEW YORK and BALLINA, Ireland, May 11, 2020 /PRNewswire/ -- Technopath Clinical Diagnostics today launched its Multichem ID-COVID-19 Quality Control Solutions offering laboratories third-party quality control products enabling an independent, totally unbiased assessment of a diagnostic device or ...
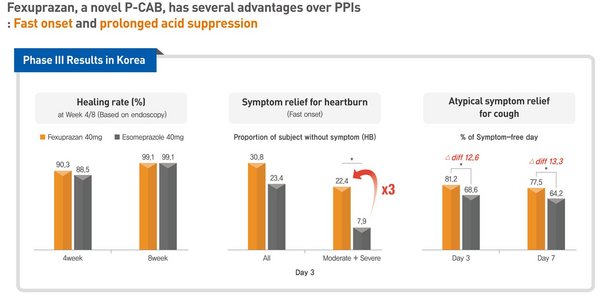
Daewoong Pharmaceutical Unveils Phase 3 Clinical Data of Fexuprazan, A Novel Potassium-competitive Acid Blocker
SEOUL, South Korea, May 11, 2020 /PRNewswire/ -- Daewoong Pharmaceutical (Daewoong) unveiled for the first time the phase 3 clinical data of Fexuprazan, a novel gastroesophageal reflux disease agent at Digestive Disease Week (DDW) 2020. The abstract of Fexuprazan has been rated in the top 10% pos...
Scientific Evidence Shows Everyday Hygiene Is Essential to Help Prevent the Spread of Infections, Reveals New Paper From The Global Hygiene Council
LONDON, May 8, 2020 /PRNewswire/ -- According to a new Position Paper published
in theAmerican Journal of Infection Control
DERMALOG Fever Detection at UKE Hamburg
HAMBURG, Germany, May 7, 2020 /PRNewswire/ -- Covid-19 currently shows how important it is to prevent the spread of infection as far as possible. Hospitals are facing a serious challenge, as staff and patients are at increased risk of infection. Fever screenings are increasingly being carried ...
Lipidor Presents Updated Project Plan - Initiates Phase III study of AKP02 in-House
STOCKHOLM, May 7, 2020 /PRNewswire/ -- In light of the company's successful Phase III study of calcipotriol spray (AKP01) against mild to moderate plaque psoriasis and subsequent discussions with pharmaceutical companies, the Board of Lipidor submits an updated project plan together with prioriti...
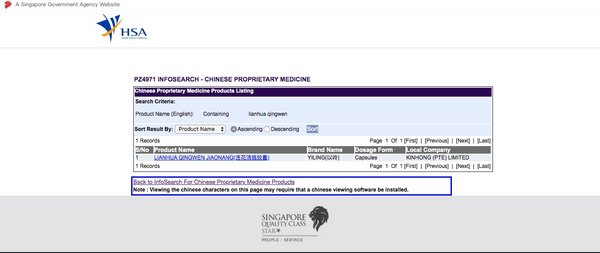
Lianhua Qingwen Receives Product Listing Approval from Singapore HSA
SHIJIAZHUANG, China, May 7, 2020 /PRNewswire/ -- Yiling Pharmaceutical (002603.SZ) announced that it has obtained the product listing approval issued by the Health Sciences Authority (HSA) ofSingapore which grants Lianhua Qingwen Capsule the initial sale authorization in the country. The approval...
TWi Biotechnology Receives Canada Health Approval for AC-1101 Phase 1 CTA
TAIPEI, Taiwan, May 7, 2020 /PRNewswire/ -- TWi Biotechnology (TWiB) announced that they have received Clinical Trial Application (CTA) approval from the Health Canada to conduct a Phase 1 clinical trial with AC-1101 gel. AC-1101 is a topical JAK inhibitor for the potential treatment of patients ...
Sai Life Sciences Joins the Global 'COVID Moonshot' Project
HYDERABAD, India, May 7, 2020 /PRNewswire/ -- Sai Life Sciences, one of India's
fastest growingContract Development & Manufacturing Organizations (CDMOs)
Tyvyt® (Sintilimab Injection) Combined with Gemzar® (Gemcitabine for Injection) and Platinum Chemotherapy Met the Predefined Primary Endpoint in the Phase 3 ORIENT-12 Study as First-Line Therapy in Patients with Locally Advanced or Metastatic Squamous Non-Small Cell Lung Cancer
SUZHOU, China, May 6, 2020 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of cancer, autoimmune, metabolic and other major diseases, today jointly...
Yiling Pharmaceutical Releases Financial Report for Q1 2020, Covid-19 TCM Lianhua Qingwen Research and Achievements Drive Growth
SHIJIAZHUANG, China, May 6, 2020 /PRNewswire/ -- Yiling Pharmaceutical (002603.SZ) has released its financial report for the first quarter of 2020. In the March quarter, Yiling Pharmaceutical achieved a total operating revenue of CNY 2.334 billion (USD $329 million) with 50.56% growth year-on-year...
Venturis Therapeutics, Inc. Announces its Applications for Four New Patents Related to the Use of Therapeutic Angiogenesis in the Treatment of Illnesses Related to the COVID-19 Virus, as Well as Illnesses Related to Other Viruses
DALLAS, May 6, 2020 /PRNewswire/ -- Venturis Therapeutics, Inc. ("VT") today announced it has applied for four provisional patents related to the treatment and/or prevention of virally induced illnesses related to the COVID-19 virus, as well as other viruses. These new patent applications cite th...
AGC Biologics Partners with Faron Pharmaceuticals to Manufacture Cancer Treatment
AGC Biologics will manufacture novel precision cancer immunotherapy treatment Clevegen SEATTLE, May 5, 2020 /PRNewswire/ -- AGC Biologics, a global Biopharmaceutical Contract Development and Manufacturing Organization (CDMO), has been selected to commercially manufacture the treatment Clevegen f...
Antengene Announces Expansion of Partnership with Karyopharm in Asia Pacific Markets
SHANGHAI, May 5, 2020 /PRNewswire/ -- Antengene Corporation (Antengene) today announced a broadened partnership and territory expansion agreement with Karyopharm Therapeutics Inc. (NASDAQ: KPTI) (Karyopharm) for development and commercialization of four oral novel drugs and drug candidates to s...
Ajinomoto Bio-Pharma Services Announces Manufacturing Partnership with CytoDyn for Drug Product Used in COVID-19 Clinical Trials
SAN DIEGO, May 5, 2020 /PRNewswire/ -- Ajinomoto Bio-Pharma Services ("Aji Bio-Pharma"), a leading provider of biopharmaceutical contract development and manufacturing services, is pleased to announce it has entered into a manufacturing services agreement with CytoDyn Inc., a late stage biotechno...
Tessa Therapeutics Announces New Executive Leadership
SINGAPORE, May 5, 2020 /PRNewswire/ -- Tessa Therapeutics (Tessa), a clinical-stage cell therapy company developing next-generation cancer treatments, today announced thatJeffrey H. Buchalter will assume the role of Chief Executive Officer, and Göran A. Ando will become Chairman of the Board, wi...
Ascentage Pharma's Core Drug Candidate HQP1351 Granted Orphan Drug Designation by the US FDA for the Treatment of Patients with Chronic Myeloid Leukemia
SUZHOU, China, and ROCKVILLE, Md., May 4, 2020 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that the US Food and Drug Adm...
Yiling Pharmaceutical to Increase Productivity of Lianhua Qingwen
SHIJIAZHUANG, China, May 4, 2020 /PRNewswire/ -- Yiling Pharmaceutical (002603.SZ) announced to launch the productivity-improving project ofLianhua Qingwen, a traditional Chinese medicine (TCM) used mainly for the treatment of colds and viral influenza. The Company is set to produce 7.5 billion c...
Reminder: Deadline To File Claims In The Purdue Pharma L.P. Bankruptcy Is June 30, 2020
NEW YORK, May 2, 2020 /PRNewswire/ -- The following is being issued by Prime Clerk, the court-appointed claims and noticing agent. This is a reminder that Tuesday, June 30, 2020, at 5:00 p.m. (Prevailing Eastern Time) is the deadline or bar date for filing claims in the bankruptcy of Purdue Phar...
Trio Pharmaceuticals, Inc. and Ajinomoto Bio-Pharma Services Enter into a Development Collaboration for a Novel Antibody Therapeutic
SAN DIEGO and SAN FRANCISCO, April 30, 2020 /PRNewswire/ -- Trio Pharmaceuticals, Inc. ("TRIO"), a cancer therapeutics company developing novel dual action antibody drugs, TRIObody™, and novel dual action antibody drug conjugates, TRIObody Drug Conjugate™ (TDC™) and Ajinomoto Bio-Pharma Services ...
Week's Top Stories
Most Reposted
Cornerstone Robotics Raises over US$70 million Funding to Forge Accessibility in Robotic Surgery
[Picked up by 323 media titles]
2025-01-13 09:05URBAN REVIVO Celebrates Lunar New Year with Unique Collaboration with Artist Mercedes Bellido
[Picked up by 294 media titles]
2025-01-13 10:44RuggON Launches VIKING II: Transforming Fleet Management with Unmatched Technology and Flexibility
[Picked up by 287 media titles]
2025-01-14 21:00Gen Z Travelers Drive Buy Now, Plan Later Travel Bookings by Over 20%: Fliggy's 2024 Buy Now Plan Later Travel Insight
[Picked up by 286 media titles]
2025-01-10 16:04VANTAGE FOUNDATION SUPPORTS GRAB INDONESIA IN EMPOWERING WOMEN DRIVER-PARTNER
[Picked up by 280 media titles]
2025-01-09 18:26