Pharmaceuticals
Ascentage Pharma Announces 2020 Annual Results and Reports Launch Readiness for Its Core Drug Candidate
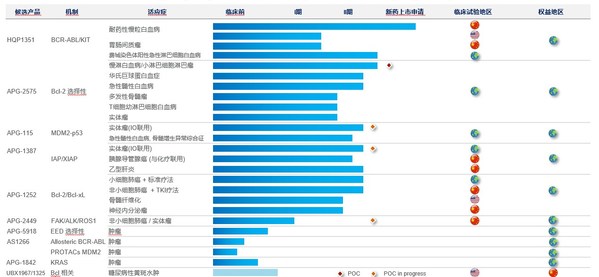
SUZHOU, China and ROCKVILLE, Md., March 31, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today released its annual results for full ...
BIOVAXYS files FDA pre-IND meeting request and briefing package for COVID-T
VANCOUVER, BC, March 31, 2021 /PRNewswire/ -- BioVaxys Technology Corp. (CSE: BIOV) (FRA:5LB) (OTC:LMNGF) ("BioVaxys"), the world leader in haptenized antigen vaccines for antiviral and cancer applications, announced today that it is has filed a pre-IND (Investigational New Drug) meeting request ...
Future Health: Innovative Health Around the World
LONDON, March 31, 2021 /PRNewswire/ -- It started with the UK but the renowned organisers of Future Health Innovation have recently announced the launch of a full series of digital events - the Health Week Series. Concentrating on key markets around the world, there will be a Summer and Autumn ev...
CStone Announces China NMPA New Drug Approval of Precision Therapy AYVAKIT® (avapritinib) for the Treatment of Adults with Unresectable or Metastatic PDGFRA Exon 18 Mutant Gastrointestinal Stromal Tumor
SUZHOU, China, March 31, 2021 /PRNewswire/ -- CStone Pharmaceuticals (CStone, HKEX: 2616), a leading biopharmaceutical company focused on developing and commercializing innovative immuno-oncology therapies and precision medicines, today announces that the National Medical Products Administration ...
Everest Medicines Announces China NMPA Approval of Clinical Trial Application to Evaluate Trodelvy® in a Phase 2 Basket Trial for a Variety of Cancers with High TROP-2 Expression
SHANGHAI, March 31, 2021 /PRNewswire/ -- Everest Medicines
Menarini Silicon Biosystems launches CELLSEARCH® Circulating Multiple Myeloma Cells Assay, a new non-invasive test to monitor disease status in clinical research studies
BOLOGNA, Italy and HUNTINGDON VALLEY, Pa., March 31, 2021 /PRNewswire/ -- Menarini Silicon Biosystems, a pioneer of liquid biopsy and single cell technologies, announced today the launch of a new assay to meet the needs of pharma companies, CROs, clinicians and research scientists working on mult...
Viva Biotech Announced 2020 Annual Results
The Group's Revenue Surged by 115.7% YoY Revenue from the CFS Business Increased Significantly by 146.3% YoY Establish One-stop Service Platform for Novel Drug Discovery and Production via Internal Growth and External Expansion Financial Highlights of the year ended December 31, 2020: * Reve...
European Commission Approves mAbxience's Bevacizumab For The Treatment Of Certain Types Of Cancer
mAbxience, a global fully-fledged biotech company, with over a decade of experience in the development, manufacture and commercialization of biopharmaceuticals, is expanding patient access to biological treatments throughoutEurope. MADRID, March 31, 2021 /PRNewswire/ -- On 31 March 2021, the Eur...
Ajinomoto Bio-Pharma Services Expands Manufacturing Agreement with AstraZeneca to Include Drug Product Manufacturing
WETTEREN, Belgium, March 31, 2021 /PRNewswire/ -- Ajinomoto Bio-Pharma Services ("Aji Bio-Pharma"), a leading provider of biopharmaceutical contract development and manufacturing services, is pleased to announce a manufacturing agreement expansion with AstraZeneca to include drug product manufact...
GemVax's highly promising Phase II Alzheimer's disease clinical trial results targeting telomerase published in prestigious Alzheimer's Research & Therapy' journal
SEOUL, South Korea, March 30, 2021 /PRNewswire/ -- GemVax & KAEL Co., Ltd. (Korea: 082270)("GemVax") has announced that a paper on GV1001, a novel Alzheimer's treatment based on telomerase modification, was published onMarch 26 th in the SCI-grade international journal 'Alzheimer's Research & Ther...
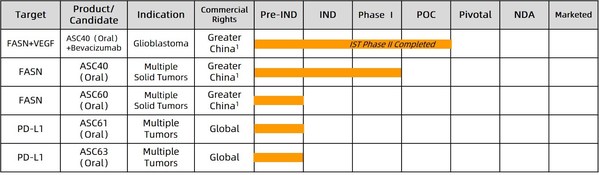
Ascletis Announces Investment Escalation in R&D of Cancer Lipid Metabolism and Oral Checkpoint Inhibitors
* Proposed Initiation of a Pivotal Phase II Trial of ASC40 in Combination with Bevacizumab in Chinese Patients with First Relapse of High-grade Astrocytoma * In-house discovered oral PD-L1 inhibitors demonstrated favorable anti-tumor activities in the animal model compared to a marketed anti-...
Novel Phenomics NASH In Vitro Assay Presented by InSphero AG and PharmaNest Inc at 2021 Society of Toxicology Meeting
Introduction of key technology to accelerate NASH drug discovery by combining 3D disease models and automated AI-based fibrosis quantification PRINCETON, New Jersey, March 30, 2021 /PRNewswire/ -- During the 2021 Society of Toxicology (SOT) Annual Meeting, researchers from Swiss-based InSphero AG...
Amplicore, Inc. Announces Closing of a Successful $4M Seed Funding Round
CINCINNATI, March 30, 2021 /PRNewswire/ -- Amplicore Inc., an early-stage biopharmaceutical company currently developing locally delivered and minimally invasive therapeutic solutions for musculoskeletal diseases, has successfully closed its Series Seed round of funding at$4 million. Investments ...
Kazia Licenses Rights to Paxalisib in Greater China to Simcere, a Leading Chinese Pharmaceutical Company
SYDNEY, March 29, 2021 /PRNewswire/ -- Kazia Therapeutics Limited (ASX: KZA; NASDAQ: KZIA), an oncology-focused drug development company, is pleased to announce that it has entered into a licensing agreement with Simcere Pharmaceutical Group Ltd (Simcere) (HKSE: 2096) to develop and commercialise...
Johnson & Johnson Announces Advance Purchase Agreement with the African Vaccine Acquisition Trust for the Company's COVID-19 Vaccine Candidate
NEW BRUNSWICK, N.J., March 29, 2021 /PRNewswire/ -- Janssen Pharmaceutica NV, one of the Janssen Pharmaceutical Companies of Johnson & Johnson (NYSE: JNJ) (the Company), has entered into an agreement with the African Vaccine Acquisition Task Team (AVATT) to make available up to 220 million dos...
Henlius Plans to File the NDA of Novel anti-PD-1 mAb HLX10 for the Treatment of MSI-H Solid Tumours, the Phase 2 Clinical Trial has Met the Primary Endpoint
SHANGHAI, March 29, 2021 /PRNewswire/ -- Shanghai Henlius Biotech, Inc. (2696.HK) announced that the Phase 2 study of its innovative PD-1 inhibitor HLX10 in patients with unresectable or metastatic microsatellite instability-high or mismatch repair-deficient (MSI-H/dMMR) solid tumors that fail t...
Making Most out of Launched Products Fueling Diversified Innovation through Integrated Platform
SHANGHAI, March 28, 2021 /PRNewswire/ -- Henlius (2696.HK) announced the financial results for the year ended31 December 2020. 2020 marked a meaningful year for Henlius as the Company gathered the pace in commercialization and continued the momentum for innovation. The Company delivered a total r...
Genscript Biotech Reports Full Year 2020 Financial Results
Highlights: * Revenue of the Group for the year ended December 31, 2020 was approximately US$390.8 million, representing an increase of 42.9% as compared with approximatelyUS$273.4 million for the year ended December 31, 2019 * Gross profit of the Group for the year ended December 31, 2...
Modern Chinese Medicine Announces Year 2020 Annual Results
Solid growth with focus to broaden the distribution network and raise R&D efforts PERFORMANCE HIGHLIGHTS * The Group posted a consolidated revenue of approximately RMB308.7 million for the year ended31 December 2020, representing an increase of approximately 41.1%. * For the year ended 31 D...
Saxenda® recommended for approval by European Medicines Agency committee for the treatment of obesity in adolescents aged 12-17 years
BAGSVÆRD, Denmark, March 26, 2021 /PRNewswire/ -- Novo Nordisk today announced that the Committee for Medicinal Products for Human Use (CHMP) under the European Medicines Agency (EMA) has recommended that the use of Saxenda® is expanded for the treatment of obesity in adolescents aged 12–17 years...
Week's Top Stories
Most Reposted
Marina Bay precinct partners UOB, Marina Bay Sands and Singapore Tourism Board, together with Disney Cruise Line, to illuminate Singapore's skyline with a fireworks sky show
[Picked up by 328 media titles]
2026-02-19 14:30Never Miss a Message: Agoda's Customer Support Now Travels With You
[Picked up by 326 media titles]
2026-02-24 12:00NextFin Asia: A New Dedicated Fund for the Catapult: Inclusion SE Asia Program to Further Scale Inclusive Finance Fintechs in ASEAN
[Picked up by 311 media titles]
2026-02-23 08:00Klook and Osaka Convention & Tourism Bureau sign MoU to advance inbound tourism and foster socio-economic development throughout Osaka Prefecture
[Picked up by 301 media titles]
2026-02-24 16:13Vitafoods Asia 2026 Expands by 30%: A Bigger, More Dynamic Trade Event with Exciting New Features & Increased International Participation
[Picked up by 288 media titles]
2026-02-23 10:09