Pharmaceuticals
Cardinal Health signs definitive agreement to sell its Cordis business to Hellman & Friedman
DUBLIN, Ohio, March 12, 2021 /PRNewswire/ -- Cardinal Health (NYSE: CAH) today announced that it has signed a definitive agreement to sell its Cordis business to Hellman & Friedman (H&F) for approximately$1 billion, which includes buyer's assumption of certain liabilities and seller's retention o...
Harbour BioMed Announces Dosing of First Patient in Tanfanercept Phase III Clinical Trial
CAMBRIDGE, Mass. and ROTTERDAM, Netherlands and SHANGHAI and SUZHOU, China, March 12, 2021 /PRNewswire/ -- Harbour BioMed (HBM) (HKEX: 02142) today announced the dosing of the first patient in the Phase III clinical trial of its new global investigational tumor necrosis factor (TNF) receptor-1 f...
Novavax Confirms High Levels of Efficacy Against Original and Variant COVID-19 Strains in United Kingdom and South Africa Trials
- 100% protection against severe disease - Final analysis in U.K. trial confirms 96% efficacy against original strain of COVID-19 - Efficacy against variants confirmed in U.K. and South Africa GAITHERSBURG, Md., March 12, 2021 /PRNewswire/ -- Novavax, Inc. (Nasdaq: NVAX), a biotechnolog...
Baxter Announces New Data on Benefits of Remote Patient Management for Home Peritoneal Dialysis World Kidney Day 11 March 2021
* Published economic modelling shows estimated reduction in hospital episodes and potential financial savings from remote patient monitoring technology. * Baxter supports World Kidney Day Steering Committee in declaring 2021 the year of "Living Well with Kidney Disease". SINGAPORE, March 11, 2...
Lianhua Qingwen -- The Leading TCM In Global Anti-Pandemic Programs
SHIJIAZHUANG, China, March 11, 2021 /PRNewswire/ -- As an epitome of the Chinese civilization, traditional Chinese medicine or TCM, and western medicine, reinforce each other to protect and improve people's health. Since the outbreak of the COVID-19 pandemic,China has been strengthening the inte...
Ascentage Pharma to Present the Latest Results from Six Preclinical Studies at AACR Annual Meeting 2021
SUZHOU, China and ROCKVILLE, Md., March 11, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that the latest preclinical...
OPTEL Group on Track for Record Year in Asia-Pacific Pharma Sales
OPTEL, a world leader in pharmaceutical track and trace for more than 30 years, expects the current fiscal year to be its best in terms of sales and project delivery to the pharmaceutical industry since the inauguration of its manufacturing plant inGoa, India, in 2016. GOA VELHA, India, March 1...
Viva Biotech Successfully Held 2021 Partnership Summit -- Novel Drug 2021, the Persistence and Transformation of Start-up Founders
SHANGHAI, March 10, 2021 /PRNewswire/ -- March 2nd-6th, 2021, Viva Biotech successfully hosted the 2021 Partnership Summit. Over 300 attendees joined the Summit, including representatives from global investment institutions, R&D heads from pharmaceutical companies, and business development leader...
Sihuan Pharmaceutical (0460.HK): Be a friend of time, 2021 is the year of turning point
HONG KONG, March 10, 2021 /PRNewswire/ -- Sihuan Pharmaceutical Holdings Group Ltd. plays a pivotal role in the domestic medical industry. It is a leading company inChina's CCV prescription drugs. According to IMS data in 2018, Sihuan Pharmaceutical is well-known on the national hospital prescrip...
Insilico's Chemistry42 AI system integrated into UCB's drug discovery programs
HONG KONG, March 9, 2021 /PRNewswire/ -- Insilico Medicine, an AI drug discovery company, announced that UCB will integrate Insilico'sChemistry42™ into UCB's internal drug discovery pipeline. UCB's early adoption of Insilico Medicine's proprietary technology will provide UCB's scientists with the...
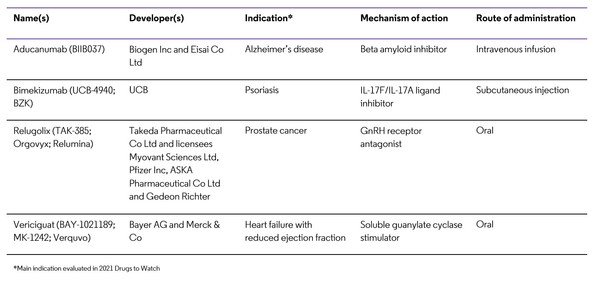
Clarivate Drugs to Watch Report Highlights Four Likely Blockbusters Among Drugs Launching in 2021
LONDON, March 9, 2021 /PRNewswire/ -- Clarivate Plc (NYSE:CLVT), a global leader in providing trusted information and insights to accelerate the pace of innovation, today announced the launch of its annual "Drugs to Watch" list, identifying drugs entering the market or launching key indications i...
Entire industrial chain resources of advanced medical equipment are lining up at Medtec China 2021
SHANGHAI, March 8, 2021 /PRNewswire/ -- The advanced medical equipment industry chain includes various fields, such as materials, design, components, parts manufacturing, and complete machine manufacturing. Each field may lead to several subdivisions, where high scientific and technological insig...
FASN Inhibitor ASC40 Demonstrates Positive Phase 2 Topline Clinical Results from China Cohort of Patients with NASH
* Oral FASN inhibitor ASC40 shown to meaningfully reduce liver fat with a 50% responder rate * Consistent improvement in biomarkers of liver inflammation as observed in U.S. cohort SHANGHAI, China and SAN MATEO, California, March 9, 2021 /PRNewswire/ -- Gannex Pharma Co., Ltd., a wholly-owned...
AMD Telemed Applauded by Frost & Sullivan for Its Modular Engage N' Solution that Supports Patient Needs by Acuity Level
AMD's unified virtual care framework connects interoperable electronic health records, medical devices, and various engagement models to offer exceptional value to clients SANTA CLARA, Calif., March 9, 2021 /PRNewswire/ -- Based on its recent analysis of the North American virtual care market,Fr...
Wugen Announces Exclusive Partnership Agreement With Alpha Biopharma in Asia For Cell Therapies to Treat Cancer
ST. LOUIS, March 8, 2021 /PRNewswire/ -- Wugen Inc., a clinical-stage biotechnology company developing novel universal natural killer (NK) and T-cell therapies for the treatment of cancer, today announced that it has entered into an exclusive license and collaboration agreement withShanghai-based...
Patients Win When Support Is Personalized: New Study From Atlantis Healthcare Shows Treatment Persistence and Adherence Improved by 38%
SYDNEY, March 8, 2021 /PRNewswire/ -- Leading patient outcomes journal Patient
Preference and Adherence
Cipla Gulf Expands Partnership with Alvotech for Commercialization of Biosimilars in Australia and New Zealand
MUMBAI, India, March 2, 2021 /PRNewswire/ -- Cipla Limited (BSE: 500087) (NSE: CIPLA EQ) referred to as "Cipla" today announced that its subsidiary, Cipla Gulf FZ LCC ("Cipla Gulf') is expanding its partnership with Alvotech for the marketing and distribution of four biosimilar medicines inAustra...
Merck and GPHL Collaborate on Business Innovation and Development in the Greater Bay Area
GUANGZHOU, China, March 2, 2021 /PRNewswire/ -- Merck, a leading science and technology company, today signed a Memorandum of Understanding (MOU) to begin strategic collaboration with Guangzhou Pharmaceutical Holdings Limited (GPHL), China's leading pharmaceutical company. The collaboration aim...
Oscotec and Beactica Therapeutics announce license and collaboration agreement to develop new cancer drug
STOCKHOLM, March 2, 2021 /PRNewswire/ -- Oscotec Inc. (039200: KOSDAQ), the Korean drug development company, and Beactica Therapeutics AB, the Swedish drug discovery company, today announced a new research development and licensing agreement. Oscotec and Beactica will initially jointly collaborat...
INOVIO Announces Positive Results from REVEAL 1, a Phase 3 Pivotal Trial Evaluating VGX-3100, its DNA-based HPV Immunotherapy for the Treatment of High-grade Precancerous Cervical Dysplasia Caused by HPV-16 and/or HPV-18
Trial achieved primary and secondary efficacy endpoints among all evaluable subjects in the Phase 3 multi-center, randomized, double-blind, placebo-controlled trial VGX-3100 is the first DNA medicine to achieve efficacy endpoints in a Phase 3 clinical trial INOVIO also continues to partner with...
Week's Top Stories
Most Reposted
Marina Bay precinct partners UOB, Marina Bay Sands and Singapore Tourism Board, together with Disney Cruise Line, to illuminate Singapore's skyline with a fireworks sky show
[Picked up by 328 media titles]
2026-02-19 14:30Never Miss a Message: Agoda's Customer Support Now Travels With You
[Picked up by 326 media titles]
2026-02-24 12:00NextFin Asia: A New Dedicated Fund for the Catapult: Inclusion SE Asia Program to Further Scale Inclusive Finance Fintechs in ASEAN
[Picked up by 311 media titles]
2026-02-23 08:00Klook and Osaka Convention & Tourism Bureau sign MoU to advance inbound tourism and foster socio-economic development throughout Osaka Prefecture
[Picked up by 301 media titles]
2026-02-24 16:13Vitafoods Asia 2026 Expands by 30%: A Bigger, More Dynamic Trade Event with Exciting New Features & Increased International Participation
[Picked up by 288 media titles]
2026-02-23 10:09