Pharmaceuticals
New Capture the Fracture® Partnership Aims for 25% Reduction in the Incidence of Hip and Vertebral Fractures Due to Osteoporosis by 2025
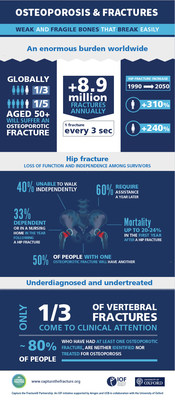
International Osteoporosis Foundation Announces First-of-its-kind Partnership WithUniversity of Oxford, Amgen and UCB to Combat Global Public Health Burden of Osteoporosis[1] Hip and Vertebral Fractures are Costly for Society and Can be Life-Altering for Patients[2],[3] NYON, Switzerland...
Investigational, Once-weekly Insulin Icodec Showed Comparable Efficacy and Safety to Once-daily Insulin Glargine U100 in Phase 2 Trial
This material is intended for global medical media only, excl US For journalistic assessment and preparation before publication. BAGSVÆRD, Denmark, June 15, 2020 /PRNewswire/ -- Today, Novo Nordisk announced results from a phase 2 clinical trial of investigational insulin icodec, a once-week...
Switching to Ozempic(R) from another GLP-1 RA significantly reduced blood sugar and weight in people with type 2 diabetes in routine clinical practice
This material is intended for global medical media only, excl US. For journalistic assessment and preparation before publication. - A separate real-world study showed that people with type 2 diabetes on two oral antidiabetic drugs who intensified with a glucagon-like peptide-1 receptor agonist ...
Harbour BioMed Appoints Dr. Jon Wigginton to its Scientific Advisory Board
Menarini Group Completes a Solid Year of Operating Performance
- Turnover reaches €3,793 million, with an increase of 3.2% compared to 2018 and EBITDA of €492 million thanks to continued strong performance of its most important products - €150 million investment into new plant in Italy significantly increasing oral blister capacity in line with increased de...
Siemens and Exyte join forces to deliver integrated solutions for fast-track construction of smart biotech facilities
Joint Press Release * Covid-19 poses major challenges for developing and mass-producing vaccines and medicines in existing production facilities * Siemens and Exyte combine their strengths to offer intelligent, modular, and scalable biotech facilities * Siemens provides solutions for proces...
L.E.A.F. Pharmaceuticals Lead Investigational Product for Lung and Colorectal Cancers, LEAF-1401, Featured at the 2020 Virtual Annual Meeting of the American Society of Clinical Oncology (ASCO)
Treatment with LEAF-1401 exposes the tumor to 20-times more pentaglutamated pemetrexed than conventional pemetrexed. Polyglutamated pemetrexed has been shown to be 80-times more potent than pemetrexed in inhibiting thymidylate synthase (TS), a key enzyme in the folate pathway. Pemetrexed (Alimta®...
Frost & Sullivan Names Top Innovators in Artificial Intelligence for Drug Discovery in the Pharmaceutical Industry
The Frost Radar benchmarks the top 16 companies in the market excelling at growth and innovation SANTA CLARA, California, June 10, 2020 /PRNewswire/ -- Frost & Sullivan's Artificial Intelligence for Drug Discovery in the Pharmaceutical Industry Radar has identified that organizations' need to mi...
WuXi Biologics Signs Lease for a Clinical Manufacturing Facility in the United States
CRANBURY, NJ and SHANGHAI, June 9, 2020 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a global company with leading open-access biologics technology platforms, announced that it has signed a 10-year lease for a clinical manufacturing facility (MFG18) inCranbury, New Jersey. The new clin...
Update: Deadline To File Claims Against Purdue Pharma L.P. Has Been Extended To July 30, 2020
NEW YORK, June 5, 2020 /PRNewswire/ -- The following is being issued by Prime Clerk LLC. In light of the ongoing COVID-19 pandemic, the U.S. Bankruptcy Court for the Southern District ofNew York has granted a 30-day extension of the deadline to file a claim against Purdue Pharma L.P.,1 including...
IPP Announces AGC Biologics Acquisition of AstraZeneca Boulder, CO Plant
PRINCETON JUNCTION, New Jersey, June 4, 2020 /PRNewswire/ -- International Process Plants (IPP), a global buyer and seller of pharmaceutical and biopharmaceutical manufacturing facilities and equipment, announced its client AGC Biologics' successful Q2/20 purchase of a state-of-the-art commercial...
Servier Completes the Acquisition of Symphogen
PARIS, June 4, 2020 /PRNewswire/ -- Servier, an independent international pharmaceutical company, today announced that it has completed the acquisition of Symphogen A/S. Symphogen will now function as Servier's antibody center of excellence across multiple therapeutic areas, including oncology. ...
Press Launch of hVIVO COVID Clear Test, the Most Accurate Antibody Test Available to UK Employers to Help Get People Back to Work
Thursday 4 June 2020, 11am at hVIVO's world leading virology clinic in Whitechapel,East London We are making available for the first time on demand antibody testing direct to large employer groups in the UK We are also seeking channel partners to make testing available through GP networks, nurs...
Novavax Selects AGC Biologics to Manufacture Matrix-M™ Adjuvant for Novel COVID-19 Vaccine
AGC Biologics partners with Novavax to produce NVX-CoV2373 component SEATTLE, June 3, 2020 /PRNewswire/ -- AGC Biologics, a global biopharmaceutical Contract Development and Manufacturing Organization (CDMO), has announced that it will partner withNovavax, Inc. (NASDAQ: NVAX), a late-stage biot...
Toni Nesci Named Head of Mayne Contract Services and New Website Launched
ADELAIDE, Australia, June 3, 2020 /PRNewswire/ -- Toni Nesci has been appointed Head of Mayne Contract Services, a division of the Mayne Pharma family. Mayne Contract Services, based in Salisbury,South Australia is the full-service pharmaceutical development and manufacturing business unit create...
Eleva's Drug Candidate to Be Examined for Use in COVID-19 Treatment
FREIBURG, Germany, June 3, 2020 /PRNewswire/ -- Eleva, a manufacturer of superior biologics, is exploring the potential of one of its drug candidates for first-in-line therapy of COVID-19. The compound, factor H, is part of the complement system, which is believed to affect the severity of the co...
Innovent Announces the Preliminary Results of the Anti-CTLA-4 Monoclonal Antibody IBI310 in a Phase 1 Clinical Study
SUZHOU, China, June 3, 2020 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of cancer, metabolic, autoimmune and other major diseases, today announces...
Technopath Clinical Diagnostics Launches the First Not-For-Profit COVID-19 Antibody Testing Initiative to Support Companies in Ireland
BALLINA (County Tipperary), Ireland, June 2, 2020 /PRNewswire/ -- Technopath Clinical Diagnostics today announced that it is rolling out COVID-19 antibody testing inIreland to help businesses navigate a safer workplace re-entry. This initiative will provide key services to all industries and will...
QPS Awarded Two 2020 CRO Leadership Awards
NEWARK, Delaware, June 2, 2020 /PRNewswire/ -- QPS is a GLP/GCP-compliant and CLIA-certified contract research organization (CRO) delivering discovery, preclinical, and clinical drug development services since 1995. An award-winning leader focused on bioanalytics and clinical trials, QPS, has be...
Innovent Announced the Preliminary Results of the Anti-PD-1/PD-L1 Bispecific Antibody IBI318 in a Phase 1 Clinical Study
SUZHOU, China, June 2, 2020 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of cancer, metabolic, autoimmune and other major diseases, announced the ...
Week's Top Stories
Most Reposted
Supported by the Luxembourg Government, The Luxembourg House of Financial Technology (LHoFT) and the Asian Development Bank (ADB) Announce the 2nd Edition of Catapult | SE Asia 2025
[Picked up by 313 media titles]
2025-01-22 15:38Blackpanda Recognized with Frost & Sullivan's Asia Pacific Company of the Year Award for Incident Response Excellence
[Picked up by 302 media titles]
2025-01-23 09:00Agoda Expands Its Eco Deals Program Pledging Up to $1.5 Million to Fund Critical Conservation Projects Across 10 Asian Markets in Partnership with WWF
[Picked up by 292 media titles]
2025-01-23 09:00Sephora Unveils Its First Film, "Beauty & Belonging" Celebrating Diversity and Authenticity
[Picked up by 288 media titles]
2025-01-24 08:00Star-Studded Sponsor Line-up Takes Centre Court at the Singapore Tennis Open 2025
[Picked up by 287 media titles]
2025-01-28 10:49