Pharmaceuticals
Himalaya Therapeutics Announces Appointment of Howe Li as Chief Medical Officer
SAN DIEGO, May 6, 2021 /PRNewswire/ -- Himalaya Therapeutics ("Himalaya"), a clinical-stage biopharmaceutical company focused on development and commercialization inGreater China of a novel class of investigational antibody therapeutics for the treatment of solid tumor cancer, which are based on ...
Everest Medicines Receives Orphan Drug Designation from the Ministry of Food and Drug Safety in South Korea for Sacituzumab Govitecan-Hziy in Metastatic Triple-Negative Breast Cancer
SHANGHAI, May 6, 2021 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK), a biopharmaceutical company focused on developing and commercializing transformative pharmaceutical products inGreater China and other parts of Asia, announced today that the Ministry of Food and Drug Safety (MFDS) inSouth...
Hope Medicine completed US$56 million in Series B financing, strengthening capabilities of global innovation
SHANGHAI, May 6, 2021 /PRNewswire/ -- Recently, the global innovative drug R&D company Hope Medicine Inc. (referred to as "HopeMed") announced the completion of a Series B financingUS$56 million. This round of financing was jointly led by Qiming Venture Partners and Grand Flight Investment. HighL...
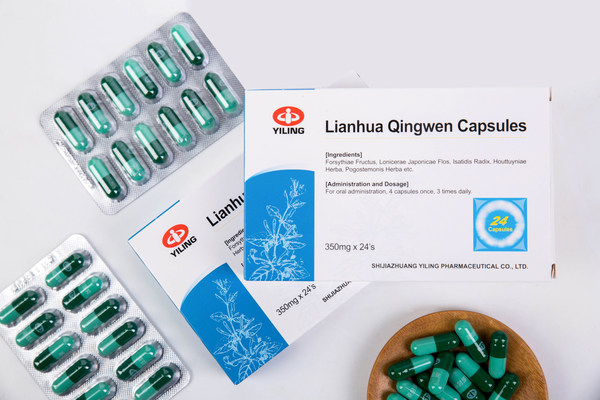
New Progress in Study on Mechanism of Lianhua Qingwen Capsules for Treatment of COVID-19
BEIJING, May 6, 2021 /PRNewswire/ -- Recently, new progress has been made in the study of the pharmacologically active ingredients and mechanism of Chinese medicine Lianhua Qingwen Capsules in the prevention and treatment of COVID-19. Lianhua Qingwen Capsules is an innovative Chinese medicine deve...
Envirotainer launches the Releye® RLP - simpler than passive, even better than active
STOCKHOLM, May 6, 2021 /PRNewswire/ -- Envirotainer, the global market leader in secure cold chain solutions for air transportation of pharmaceuticals, today launched their latest innovation, the Releye® RLP, a temperature-controlled air freight container with a revolutionary new footprint, unsur...
Yangtze River Pharmaceutical Group Selects TraceLink Platform for Global Compliance
NORTH READING, Mass., May 5, 2021 /PRNewswire/ -- TraceLink Inc., the leading digital network platform company, today announced that Yangtze River Pharmaceutical Group has selected TraceLink's global compliance solution to aid its plans for global growth. As the company gears up for international...
CSafe Global Providing Thermal Protection for COVID-19 Vaccine Shipments from BioNTech Facility
The tailor-made double-wall VIP insulated shipper provides 10+ days of temperature protection for vaccine shipments from BioNTech's facility with minimal dry ice. DAYTON, Ohio, May 5, 2021 /PRNewswire/ -- CSafe Global, the innovation leader in active, passive parcel and cell and gene temperature...
Galmed Announces Approval of IND Application in China for Aramchol for the Treatment of NASH & Fibrosis in the Global Phase 3 ARMOR Registrational Study
China to Join Galmed's Phase 3 ARMOR Registrational Study for the Treatment of NASH & Fibrosis TEL AVIV, Israel, May 3, 2021 /PRNewswire/ -- Galmed Pharmaceuticals Ltd. (Nasdaq: GLMD) ("Galmed" or the "Company"), a clinical-stage biopharmaceutical company for liver, metabolic and inflammatory di...
BioVaxys And BioElpida Sign Definitive Exclusive Agreement To Begin Ovarian Cancer Vaccine Bioproduction
VANCOUVER, BC, May 3, 2021 /PRNewswire/ -- BioVaxys Technology Corp. (CSE: BIOV) (CSE: BIOV.CN) (FRA: 5LB) (OTC: BVAXF) ("BioVaxys" or "Company") announced today that it has signed the definitive exclusive bioproduction agreement ("Agreement") with BioElpida S.A.S. ("BioElpida") of Lyon,Franc...
Asclepius Meditec's Hydrogen Oxygen Generator with Nebulizer is Expected to Alleviate the Oxygen Shortage Crisis during COVID-19 Pandemic
SHANGHAI, May 1, 2021 /PRNewswire/ -- In June, 2020, an academic paper on patients with COVID-19 inhaling hydrogen oxygen mixed gas produced by Asclepius Meditec's Hydrogen Oxygen Generator with Nebulizer was published in the Journal of Thoracic Disease. The multicenter, and open-label clinical t...
BioVaxys Broadens Intellectual Property Portfolio Commercial Trademark Application Filed for CoviDTH(R) Diagnostic
Commercial trademark application FILED for CoviDTH® diagnostic cancer vaccine platform patent coverageexpanded to NOW include over 12 tumor types VANCOUVER, B.C., April 29, 2021 /PRNewswire/ -- BioVaxys Technology Corp. (CSE: BIOV) (FRA: 5LB) (OTC: BVAXF) ("BioVaxys" or "the Company"), the world ...
Hilco Industrial Acquires All Machinery & Equipment from Legacy Pharmaceutical Switzerland
BASEL, Switzerland, April 29, 2021 /PRNewswire/ -- Hilco Industrial Acquisitions, anAmsterdam-based Industrial Asset Disposition and Acquisition Company, acquired all Machinery & Equipment from theSwiss-based CMO Legacy Pharmaceuticals. After several years of expansion, the Swiss company was forc...
Alphamab announced PD-L1/CTLA-4 bispecific antibody KN046 will enter new Phase II clinical trial in combination with Pfizer's Inlyta® (axitinib)
SUZHOU, China, April 29, 2021 /PRNewswire/ -- Alphamab Oncology (stock code: 9966HK) announced that it has entered into a clinical trial collaboration and supply agreement with Pfizer to evaluate the efficacy and safety of KN046 in combination with Inlyta® (axitinib) for the first-line treatment ...
Innovent Announces First Patient Dosed in the Phase 2 Clinical Trial of IBI302, a First-in-class Ophthalmic Anti-VEGF and Anti-Complement Bispecific Fusion Protein for Neovascular Age-Related Macular Degeneration
SAN FRANCISCO and SUZHOU, China, April 29, 2021 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, metabolic, autoimmune, ophthalmology an...
AnchorDx Jointly Publishes Clinical Research Paper: "Accurate Diagnosis of Pulmonary Nodules Using a Non-invasive DNA Methylation Test"
GUANGZHOU, China, April 28, 2021 /PRNewswire/ -- AnchorDx, a world-leading developer of cancer screening and early detection solutions, together with renowned thoracic experts from 14 top-tier hospitals inChina, jointly published a clinical research paper entitled "Accurate Diagnosis of Pulmonary...
Novaliq Announces More than 50% Patients Enrolled in CyclASol® Phase 3 Dry Eye Disease Trial ESSENCE-2
- Topline results expected in 2nd half 2021; 475 out of a 834 target patients randomized in ESSENCE-2 - Enrollment for 12-month safety extension trial (ESSENCE-2 OLE) completed - Phase 2B/3 Trial ESSENCE-1 Published in Cornea: The Journal of Cornea and External Disease HEIDELBERG, Germany and C...
CROs dMed and Clinipace Merge to Accelerate Customer Success
SHANGHAI and MORRISVILLE, N.C., April 28, 2021 /PRNewswire/ -- dMed Global, a full-service Clinical Contract Research Organization (CRO) based inShanghai, China, and Clinipace Incorporated, a full-service Clinical CRO with headquarters inNorth Carolina, USA, announced today that the two companies...
Hovid Berhad Partners with Esker to Automate its Accounts Payable Process
SINGAPORE, April 27, 2021 /PRNewswire/ -- Esker
DiscGenics Completes Patient Enrollment in Japanese Clinical Study of Cell Therapy for Disc Degeneration as US Study Achieves One-Year Follow-up Milestone
SALT LAKE CITY and TOKYO, April 27, 2021 /PRNewswire/ -- DiscGenics, Inc., a clinical stage biopharmaceutical company focused on developing regenerative cell-based therapies that alleviate pain and restore function in patients with degenerative diseases of the spine, today announced that it has c...
Himalaya Therapeutics Announces Appointment of Brian Zhang as Chief Executive Officer
SAN DIEGO, April 26, 2021 /PRNewswire/ -- Himalaya Therapeutics ("Himalaya"), a clinical-stage biopharmaceutical company focused on development and commercialization inGreater China of a novel class of investigational antibody therapeutics for the treatment of solid tumor cancer, which are based ...
Week's Top Stories
Most Reposted
Edufrienz 99: One of Asia's First Digital Platform Advancing Character Education for Future-Ready, Compassionate Children
[Picked up by 316 media titles]
2026-01-23 11:03UOB prices AUD2 billion in five-year senior unsecured notes
[Picked up by 305 media titles]
2026-01-23 09:00AVPN's AI Opportunity Fund Expands Regional Efforts to Build AI Skilling Infrastructure for a Future-Ready Workforce Across Asia-Pacific
[Picked up by 299 media titles]
2026-01-21 12:39Government of Telangana and Blaize Sign MoU at Davos to Launch Telangana AI Innovation Hub and Advance Applied AI Initiatives
[Picked up by 297 media titles]
2026-01-22 15:16Klook's study of 374,000 reviews finds that lesser-known cities create deeper emotional connection with travelers
[Picked up by 280 media titles]
2026-01-20 15:40