Pharmaceuticals
Envirotainer Opens First RAP e2 Station in Taipei
UPPLANDS VÄSBY , Sweden, Jan. 21, 2021 /PRNewswire/ -- Envirotainer, the global market leader and by far the largest provider of secure cold chain solutions for air transport of pharmaceuticals, today announced that they are expanding their network of RAP e2 stations by addingTaipei (TPE) to its ...
Merck Announces Update on the INTR@PID Clinical Program Including Lung 037 Study
DARMSTADT, Germany, Jan. 20, 2021 /PRNewswire/ -- Not intended for US-, Canada- or UK-based media Merck, a leading science and technology company, today announced an update on the Phase III INTR@PID Lung 037 study and the extensive INTR@PID clinical trial program for the potential first-in-class...
Clarivate Enhances Cortellis CMC Intelligence Platform with Addition of Comprehensive Biologics Content
LONDON, Jan. 20, 2021 /PRNewswire/ -- Clarivate Plc (NYSE: CCC), a global leader in providing trusted information and insights to accelerate the pace of innovation, today announced the enhancement ofCortellis CMC Intelligence™, the world's only organized, timely and accurate source of Chemistry, ...
Innovent Announces an Out-license Agreement with PT Etana Biotechnologies Indonesia to Launch BYVASDA® (Bevacizumab Biosimilar) in Indonesia
SAN FRANCISCO and SUZHOU, China, Jan. 19, 2021 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of cancer, metabolic, autoimmune and other major diseas...
Tessa Therapeutics Receives PRIME Designation from European Medicines Agency for CD30 CAR-T Therapy
BEDMINSTER, N.J. and SINGAPORE, Jan. 18, 2021 /PRNewswire/ -- Tessa Therapeutics (Tessa), a clinical-stage cell therapy company developing next-generation cancer treatments for hematological malignancies and solid tumors, today announced that the European Medicines Agency (EMA) has granted PRior...
Everest Medicines Announces Amended Agreement with Spero Therapeutics
SHANGHAI, Jan. 18, 2021 /PRNewswire/ -- Everest Medicines, a biopharmaceutical company focused on developing and commercializing transformative pharmaceutical products that address critical unmet medical needs for patients inGreater China and other parts ofAsia, today announced that under an amen...
Modern Chinese Medicine Group Co., Ltd. announces its subscription results
HONG KONG, Jan. 14, 2021 /PRNewswire/ -- Modern Chinese Medicine Group Co., Ltd. ("Modern Chinese Medicine", together with its subsidiaries, the "Group"; stock code: 1643), which principally engages in the production of proprietary Chinese medicine (the "PCM") and offers both over-the-counter (the...
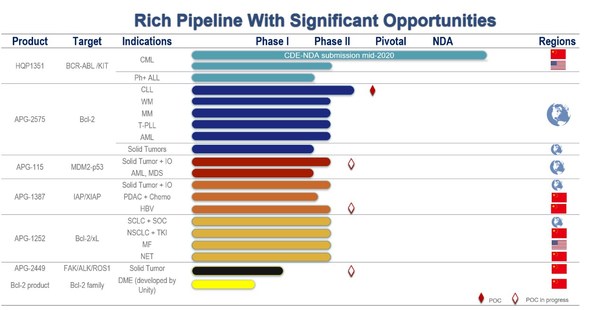
Ascentage Pharma Presents Updates on its Global Clinical Development at the J.P. Morgan 39th Annual Healthcare Conference
SUZHOU, China and ROCKVILLE, Md., Jan. 14, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, presented at the J.P. Morgan 39th Annual Hea...
Hanmi presents solution package for coping with COVID-19, including the mass production of nucleic acid vaccines (DNA/mRNA vaccine)
SEOUL, South Korea, Jan. 13, 2021 /PRNewswire/ -- Hanmi Pharmaceutical Co., Ltd., announced "endless challenges and new commitments for the pharmaceutical industry" as their management slogan for the new year, presenting the business direction for the year 2021 at the 39th JP Morgan Healthcare...
Innovent Announces NMPA Acceptance of a Supplemental New Drug Application for TYVYT® (sintilimab injection) in Combination with BYVASDA® (bevacizumab injection) as First-Line Therapy in Hepatocellular Carcinoma (HCC)
SAN FRANCISCO and SUZHOU, China, Jan. 13, 2021 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of cancer, metabolic, autoimmune and other major diseas...
Kira Pharmaceuticals to Present at the 39th Annual J.P. Morgan Healthcare Conference
CAMBRIDGE, Mass. and SUZHOU, China, Jan. 12, 2021 /PRNewswire/ -- Kira Pharmaceuticals, a global biotechnology company pioneering a new generation of complement-targeted therapies to treat immune-mediated diseases, today announced thatFrederick Beddingfield, Chief Executive Officer of Kira Pharm...
Beactica Therapeutics and University of Dundee announce collaboration to develop WRN inhibitors to target cancer
STOCKHOLM, Jan. 12, 2021 /PRNewswire/ -- Beactica Therapeutics AB, the Swedish drug discovery company, andUniversity of Dundee, a top-ranked university in the UK for biological sciences, today announced a new research collaboration agreement. The two parties will work together in a project aimed ...
Valo Raises $190 Million in Series B Financing and Unveils Select Therapeutic Programs
Details of Initial Therapeutic Programs to be Shared at the 39th J.P. Morgan Healthcare Conference BOSTON, Jan. 11, 2021 /PRNewswire/ -- Valo Health LLC (Valo), the technology company working to transform the drug discovery and development process and accelerate the creation of life-changin...
Gannex Announces Positive Phase I Clinical Results on Its THR-β Agonist ASC41
SHANGHAI, Jan. 11, 2021 /PRNewswire/ -- Gannex, a wholly owned company of Ascletis Pharma Inc. (HKEX: 1672) and fully dedicated to the R&D and commercialization of new drugs in the field of non-alcoholic steatohepatitis (NASH), today announces the positive phase I clinical results of ASC41 oral ...
MedSkin Solutions Dr. Suwelack AG Receives FDA 510(k) Clearance for MatriDerm® - Its Three-Dimensional Acellular Collagen Elastin Dermal Matrix Portfolio
MedSkin Solutions Dr. Suwelack AG announces receiving FDA 510(k) clearance for its flagship Med Care product portfolio MatriDerm®. HAMBURG, Germany, Jan. 12, 2021 /PRNewswire/ -- MatriDerm® is a single-use three-dimensional acellular dermal matrix composed of bovine collagen fibers and bovine el...
Regent Pacific Announces Key Research Findings On FORTACIN™
HONG KONG, Jan. 11, 2021 /PRNewswire/ -- Regent Pacific Group Limited ("Regent Pacific" or the "Company" and together with its subsidiaries, the "Group"; stock code: 0575.HK), a specialist healthcare, wellness and life sciences investment group is pleased to announce several key findings on the w...
Pivotal phase III clinical trial IND submission of the original innovative anticancer drug Chiauranib for the treatment of small cell lung cancer was accepted by the Center of Drug Evaluation of the NMPA.
SHENZHEN, China, Jan. 11, 2021 /PRNewswire/ -- On January 8, 2021, Shenzhen Chipscreen Biosciences Co., Ltd. (Chipscreen Biosciences, Stock Symbol: 688321.SH) submitted its pivotal phase III clinical trial application (IND) of national class I innovative drug Chiauranib to NMPA, for the treatment...
Antengene to Present at the 39th Annual J.P. Morgan Healthcare Conference
SHANGHAI and SAN FRANCISCO, Jan. 11, 2021 /PRNewswire/ -- Antengene Corporation Limited ("Antengene", SEHK: 6996.HK), a leading innovative biopharmaceutical company dedicated to discovering, developing and commercializing global first-in-class and/or best-in class therapeutics in hematology an...
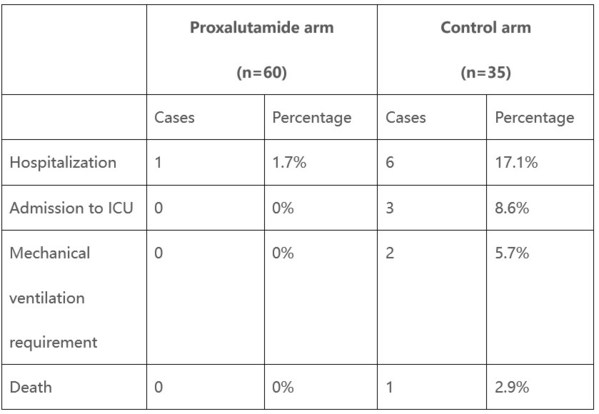
Kintor's Proxalutamide (GT0918) COVID-19 Clinical Trial Shows Positive Preliminary Results in Treatment of Female Patients
SUZHOU, China, Jan. 10, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited (HKEX: 9939) is pleased to update the preliminary analysis of the clinical trial of Proxalutamide for the treatment of COVID-19 patients. The investigator initiated trial conducted by Dr.Andy Goren and Dr. Flávio Adsuara C...
NTC grants to Zhaoke exclusive license and distribution rights in Asia for NTC014, innovative drug for bacterial conjunctivitis
MILAN and HONG KONG, Jan. 8, 2021 /PRNewswire/ -- NTC, an international R&D focused pharmaceutical company headquartered inItaly, and Zhaoke (Hong Kong) Ophthalmology Pharmaceuticals Limited, having a fully integrated ophthalmic platform with strong foundation in eyecare, announce today their agr...
Week's Top Stories
Most Reposted
k-ID Closes $45 Million Series A from Andreessen Horowitz and Lightspeed Venture Partners to Set a New Global Benchmark for Age-appropriate Gaming Experiences
[Picked up by 320 media titles]
2024-06-25 21:0010 Start-ups Awarded in HK Tech 300 Southeast Asia Start-up Competition Fostering Innovation & Entrepreneurship Beyond Boundaries
[Picked up by 316 media titles]
2024-06-27 18:56Labuan IBFC Inc. and STEP Malaysia jointly host wealth management and estate planning event
[Picked up by 314 media titles]
2024-06-28 12:45Born Global, Connecting the World - C&D Inc. Debuts at the "The 1st Global Summit of Chinese Enterprises Going Overseas and 2024 Mid-Year Industry Summit"
[Picked up by 297 media titles]
2024-06-23 13:50SCO strengthening youth development for better future
[Picked up by 297 media titles]
2024-06-24 10:52