Pharmaceuticals
Antengene Announces Approval of IND Application in China for a Phase 3 Clinical Trial of ATG-010 (Selinexor) in Combination with Bortezomib and Dexamethasone (SVd) for the Treatment of rrMM
SHANGHAI and HONG KONG, Dec.18, 2020 /PRNewswire/ -- Antengene Corporation Limited ("Antengene", SEHK: 6996.HK), a leading innovative biopharmaceutical company dedicated to discovering, developing and commercializing global first-in-class and/or best-in class therapeutics in hematology and oncolo...
Research on preventative nasal spray, which protects against COVID-19 and common cold, published in leading peer-reviewed academic journals
* Research on preventative COVID-19 nasal spray published in prestigious, peer-reviewed academic journals: EBioMedicine and the European Respiratory Journal * Novel therapy developed by Australian biotech company, Ena Respiratory, shown in animal study to reduce COVID-19 virus levels in the n...
dMed Completed a US$100 Million Series C Financing
SHANGHAI, Dec. 17, 2020 /PRNewswire/ -- dMed, the clinical CRO with strong presence inChina and the US, the world's two largest pharmaceutical markets and drug development sites, announced today its successful completion of aUS$100 million Series C financing. Led by Fidelity Management & Research...
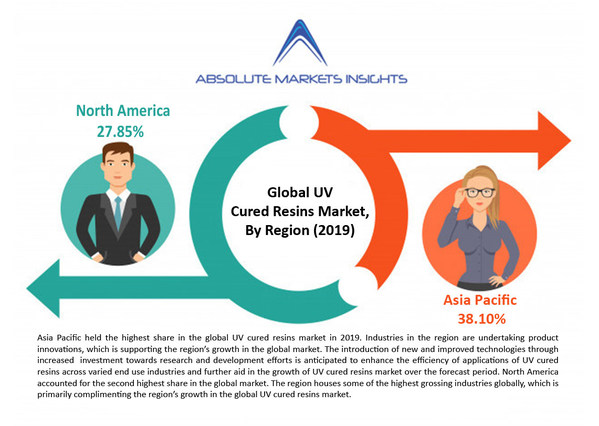
Global UV Cured Resins Market will grow to US$ 7892.70 Mn by 2028 at 8.9% CAGR - says Absolute Markets Insights
PUNE, India, Dec. 17, 2020 /PRNewswire/ --
Harbour BioMed Completes Its Global Offering and Listing on the Hong Kong Stock Exchange
CAMBRIDGE, Mass. and SUZHOU, China, Dec. 17, 2020 /PRNewswire/ -- HBM Holdings Limited ("Harbour BioMed", "HBM" or the "Company"; Stock Code: 02142.HK), a global clinical-stage biopharmaceutical company, completed its global offering (the "Global Offering") and its listing on the Main Board of ...
Covance, LabCorp's Drug Development Business, Acclaimed by Frost & Sullivan for Its Unmatched Breadth of Services to Support Clinical Trials
LONDON, Dec. 16, 2020 /PRNewswire/ -- Based on its recent analysis of the global contract research organization (CRO) market, Frost & Sullivan recognizes LabCorp's drug development business, Covance, with the 2020 Global CRO Company of the Year Award. The company has built technology-enabled solu...
EpiVax Reports Record Year for Immunogenicity Screening Toolkit (ISPRI)
PROVIDENCE, R.I., Dec. 16, 2020 /PRNewswire/ -- EpiVax, Inc. reports a record-breaking year for the "ISPRI" immunogenicity screening toolkit. More pharmaceutical and biotechnology companies signed new and continuing licenses for access to the toolkit in 2020 than ever before in the company's twen...
RedHill's Phase 2/3 COVID-19 Candidate Opaganib Reduces ARDS-Related Blood Clotting in Preclinical Model
Acute respiratory distress syndrome (ARDS)-induced thrombosis (blood clotting) may occur in up to one-third of COVID-19 patients requiring ICU admission and is a contributing cause of mortality Opaganib demonstrated reduced thrombosis in a preclinical model of ARDS Opaganib has also been shown t...
ADC Therapeutics and Overland Pharma Form Strategic JV to Expand ADC Drugs Development and commercialization in Greater China and Singapore, Advised by MSQ Ventures
NEW YORK, Dec. 15, 2020 /PRNewswire/ -- M.S.Q. Ventures ("MSQ") is pleased to announce that its client, ADC Therapeutics SA (NYSE: ADCT), has successfully entered into an agreement to jointly form a new company, Overland ADCT BioPharma (CY) Limited with Overland Pharmaceuticals, a fully integrate...
PCCS Group Has Reached a Strategic Cooperation With Shenqi Medical to Seek a Grand Blueprint of Medical Health in the Asia-pacific Region
SHANGHAI, Dec. 15, 2020 /PRNewswire/ -- Malaysia's PCCS Group Berhad (hereinafter referred to as "PCCS") andChina's Shanghai Shenqi Medical Co., Ltd. (hereinafter referred to as "Shenqi Medical") today announced the signing of a memorandum of understanding (MOU) to cooperatively pursue the potent...
Harbour BioMed and Utrecht University Announce License Agreement with AbbVie and Initiation of COVID-19 Antibody Clinical Trials
CAMBRIDGE, Mass., SUZHOU, China, UTRECHT, Netherlands, Dec. 15, 2020 /PRNewswire/ -- Harbour BioMed (HBM)(HKEX:02142), andUtrecht University (UU) today announced that they licensed to AbbVie their fully human, SARS-CoV-2 neutralizing antibody, 47D11 and program, for the prevention and treatment o...
Regent Pacific's Commercial Strategic Partner Receives Approval from Taiwan Food and Drug Administration
HONG KONG, Dec. 14, 2020 /PRNewswire/ -- Regent Pacific Group Limited ("Regent Pacific" or the "Company" and together with its subsidiaries, the "Group"; stock code: 0575.HK), a specialist healthcare, wellness and life sciences investment group is pleased to announce that Orient EuroPharma Co., L...
The EspeRare Foundation and Pierre Fabre join forces to develop and market a pioneering treatment for XLHED, a dermatologic-related rare genetic disease that requires prenatal therapeutic intervention
GENEVA and CASTRES , France, Dec. 14, 2020 /PRNewswire/ -- The EspeRare Foundation and the Pierre Fabre group announced today that they have entered into a license and development collaboration agreement for the development and commercialization of ER-004, a prenatal treatment for XLHED (X-linked...
Gannex Received U.S. FDA Fast Track Designation for Its NASH Drug Candidate ASC42,an FXR Agonist
SHANGHAI, Dec. 14, 2020 /PRNewswire/ -- Gannex, a wholly owned company of Ascletis Pharma Inc. (HKEX:1672) and fully dedicated to the R&D and commercialization of new drugs in the field of NASH, announced today that it received Fast Track designation from the U.S. Food and Drug Administration (F...
Kintor Proxalutamide's COVID-19 Clinical Trial Shows Significant Reduction in Hospitalization and Ventilation Rates
SUZHOU, China, Dec. 12, 2020 /PRNewswire/ -- Kintor Pharmaceutical Limited (HKEX: 9939) is pleased to update the preliminary analysis of the Proxalutamide's clinical trial for the treatment of COVID-19. Proxalutamide is a new androgen receptor (AR) antagonist developed in by Kintor Pharmaceutica...
BAVENCIO® (avelumab) Receives Positive CHMP Opinion for First-Line Maintenance Treatment of Locally Advanced or Metastatic Urothelial Carcinoma
Not intended for US-, Canada- and UK-based media DARMSTADT, Germany and NEW YORK, Dec. 11, 2020 /PRNewswire/ -- Merck and Pfizer Inc. (NYSE: PFE) today announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion rec...
Kintor Pharmaceutical Announces Preliminary Results from the Proxalutamide's Clinical Trial
SUZHOU, China, Dec. 11, 2020 /PRNewswire/ -- Kintor Pharmaceutical Limited (HKEX: 9939) is pleased to update the preliminary analysis of the Proxalutamide's clinical trial for COVID-19. Proxalutamide is a new androgen receptor (AR) antagonist developed in by Kintor Pharmaceutical and is currentl...
Innovent Announces First Patient Dosed in Phase 2 Pivotal Trial of IBI310 (CTLA-4) combined with TYVYT® (sintilimab injection) for the treatment of second-line or above Advanced Cervical Cancer
SAN FRANCISCO and SUZHOU, China, Dec. 11, 2020 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, metabolic, autoimmune and other major di...
Antengene Announces Acceptance of IND Application in China for a Phase 3 Clinical Trial of ATG-010 (Selinexor) in Combination with Bortezomib and Dexamethasone (SVd) for the Treatment of rrMM
SHANGHAI and HONG KONG, Dec. 11, 2020 /PRNewswire/ -- Antengene Corporation Limited ("Antengene", SEHK: 6996.HK), a leading innovative biopharmaceutical company dedicated to discovering, developing and commercializing global first-in-class and/or best-in class therapeutics in hematology and oncol...
European Union Intellectual Property Office Revokes Counterfeiter's Fake AQUAGOLD® Design Registration and Orders Payment to Aquavit of All Legal Fees and Costs
NEW YORK, Dec. 11, 2020 /PRNewswire/ -- Aquavit Pharmaceuticals, Inc. ("Aquavit") received a decision from the European Union Intellectual Property Office (EUIPO) today that it has revoked the counterfeiter U-BioMed's falsely registered AQUAGOLD® design, originally created and owned by Aquavit. ...
Week's Top Stories
Most Reposted
Another 'Reason to Travel': Agoda and Singapore Tourism Board Renew Partnership to Boost Travel to Singapore
[Picked up by 350 media titles]
2024-06-19 11:00MIFB 2024 ENLISTS F&B EXPERTS TO SPEAK ON THE LATEST IN INDUSTRY TRENDS AND THE FUTURE OF FOOD
[Picked up by 335 media titles]
2024-06-20 16:24New Study Finds Cybersecurity as Top Concern Among Automotive Manufacturers
[Picked up by 317 media titles]
2024-06-19 08:05k-ID Closes $45 Million Series A from Andreessen Horowitz and Lightspeed Venture Partners to Set a New Global Benchmark for Age-appropriate Gaming Experiences
[Picked up by 317 media titles]
2024-06-25 21:00THEIR ROYAL HIGHNESSES THE DUKE AND DUCHESS OF EDINBURGH CELEBRATE THE GRAND OPENING OF THE PENINSULA LONDON, BELGRAVIA
[Picked up by 313 media titles]
2024-06-19 11:38