Biotechnology
Portfolio Highlights: Clinical and Financial Updates of ABM, F5, AceLink, HAYA, Regenacy, and Arthrosi
SHANGHAI, Sept. 28, 2022 /PRNewswire/ -- As the investment division of Viva Biotech, Viva BioInnovator is committed to being a collaborative platform for Innovative Biotech companies from around the world. Over the past 2 months, our portfolio companies progressed greatly. ABM Therapeutics Annou...
The 6th China (Shenzhen) Innovation & Entrepreneurship International Competition Eindhoven, Netherlands Division is Open for Registration
DIGITAL ACCESS TO THE WORLD CREATES A BETTER FUTURE EINDHOVEN, Netherlands, Sept. 28, 2022 /PRNewswire/ -- China (Shenzhen)Innovation and Entrepreneurship Competition, known as the "Olympics" of innovation and entrepreneurship circle, is here again! The sixth International competition was offici...
Harbour BioMed Announces First Subject Dosed in Phase I Study of Next-Gen Anti-TSLP Fully Human Monoclonal Antibody
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, Sept. 27, 2022 /PRNewswire/ -- Harbour BioMed ("HBM", HKEX: 02142) today announces that it has completed the first subject dosing in a phase I study of HBM9378 (or SKB378 as referred to by Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd...
Bioheng Biotech Announces Publication of Impressive Results with CD7-targeted allogeneic CAR-T cell therapy for Relapsed or Refractory T Cell Malignancies
NANJING and HANGZHOU, China, Sept. 26, 2022 /PRNewswire/ -- Nanjing Bioheng Biotech Co., Ltd, a clinical-stage biotechnology company focused on developing novel cellular immunotherapy, today announced that a phase I clinical study results of RD13-01, an anti-CD7 universal CAR-T therapy product, h...
PharmAbcine to Participate in BIO-Europe 2022
DAEJEON, South Korea, Sept. 26, 2022 /PRNewswire/ -- PharmAbcine Inc. (KOSDAQ: 208340ks), a clinical-stage biotech company focusing on the development of next generation antibody therapeutics, announced today that the Company will participate in the upcoming BIO-Europe 2022 which will take place ...
WuXi STA Opens First High Potency Oral Drug Product Manufacturing Facility
New facility expands integrated drug product R&D and manufacturing services to global customers SHANGHAI, Sept. 26, 2022 /PRNewswire/ -- WuXi STA, a leading global Contract Research, Development, and Manufacturing Organization (CRDMO), today announced the opening of its first high potency (HP) o...
CellOrigin Biotech Announces Global Strategic Collaboration with Qilu Pharma to Develop "Off-the-Shelf" CAR-iMAC Cell Therapy
HANGZHOU, China, Sept. 26, 2022 /PRNewswire/ -- CellOrigin Biotech (Hangzhou) Co., Ltd. announced it has made a global strategic collaboration agreement with Qilu Pharma to develop, manufacture and commercialize proprietary "off-the-shelf" induced pluripotent stem cell- (iPSC) derived Chimeric An...
Ascentage Pharma Announces Phase I/II Data of Olverembatinib (HQP1351) Published in the Journal of Hematology & Oncology, Further Validating the Drug's Best-in-Class Potential
SUZHOU, China and ROCKVILLE, Md., Sept. 21, 2022 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, announced that the results from a Phase I and Phase II study of ...
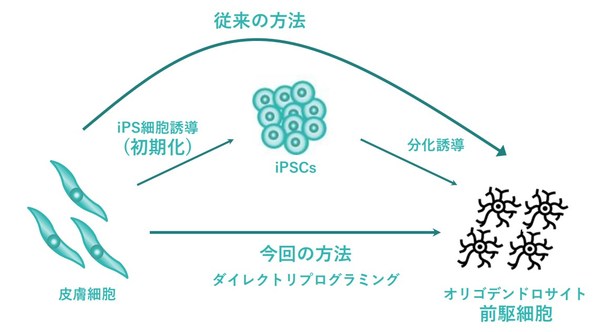
GMP cell CDMO I Peace, Inc. in collaboration with universities successfully converted human dermal fibroblasts into oligodendrocyte by direct reprogramming
PALO ALTO, Calif., Sept. 20, 2022 /PRNewswire/ -- Koji Tanabe of I Peace, Inc. (
https://www.ipeace.com
Alterity Therapeutics Receives U.S. FDA Approval for Investigational New Drug Application for ATH434 for the Treatment of Multiple System Atrophy
Regulatory Authorization Granted to Proceed with ATH434 Phase 2 Clinical Trial MELBOURNE, Australia and SAN FRANCISCO, Sept. 20, 2022 /PRNewswire/ -- Alterity Therapeutics (ASX: ATH, NASDAQ: ATHE) ("Alterity" or "the Company"), a biotechnology company dedicated to developing disease modifying tr...
Special interview with Dr. Patrick Yu-Wai-Man on International LHON Awareness Day
WUHAN, China and SAN DIEGO, Sept. 19, 2022 /PRNewswire/ -- September 19th is International LHON Awareness Day. LHON is a debilitating disease that leads to quick vision loss concomitantly or successively. It mostly impacts young adolescent males and brings tremendous physical and psychological st...
Taiwan's Anyong Biotechnology First To Employ Seafood Stem-Cell Freezing
KAOHSIUNG, Taiwan, Sept. 19, 2022 /PRNewswire/ -- Anyong Biotechnology, the world-class aquatic food processing company based inTaiwan, is now the first company in the region to employ Cells Alive System (CAS) technology fromJapan. It ranks as the most advanced freezing technology inAsia and is a...
New treatment for rare cancer cholangiocarcinoma approved in Australia
* Available to treat adults with locally advanced or metastatic cholangiocarcinoma with an FGFR2 fusion or rearrangement * PEMAZYRE® (pemigatinib) is available via a co-pay access program in Australia SINGAPORE, Sept. 15, 2022 /PRNewswire/ -- A NEW targeted therapy to treat a rare bile duct c...
Hexvix®, A Diagnostic Drug of Asieris, is Now Covered by the 2022 Lecheng Global Specialty Drug Insurance
SHANGHAI, Sept. 15, 2022 /PRNewswire/ -- Asieris Pharmaceuticals (688176.SH), a global biopharma company specializing in discovering, developing and commercializing innovative drugs for the treatment of genitourinary tumors and other related diseases, announced today that Hexvix®, a drug used for...
Gracell Biotechnologies to Participate in Three Upcoming Investor Conferences
SAN DIEGO, Calif., and SUZHOU and SHANGHAI, China, Sept. 14, 2022 /PRNewswire/ -- Gracell Biotechnologies Inc. (NASDAQ: GRCL) ("Gracell"), a global clinical-stage biopharmaceutical company dedicated to discovering and developing highly efficacious and affordable cell therapies for the treatment ...
DR CATHERINE MOHR TO JOIN BOARD OF MEDTECH COMPANY AROA BIOSURGERY
AUCKLAND, New Zealand, Sept. 14, 2022 /PRNewswire/ -- New Zealand based soft
tissue regeneration company Aroa Biosurgery (AROA) is delighted to announce the
appointment of award-winning medical researcher and inventor, Dr.Catherine Mohr
as a Non-Executive Director to the board.
MD ANDERSON AND RADIOPHARM THERANOSTICS LAUNCH JOINT VENTURE TO DEVELOP NOVEL RADIOPHARMACEUTICALS
MELBOURNE, Australia, Sept. 13, 2022 /PRNewswire/ -- The University of Texas MD
Anderson Cancer Center
GBB Lands USD 15M Pre-Series B Funding Led by Tiger Jade Capital
HONG KONG, Sept. 13, 2022 /PRNewswire/ -- Great Bay Bio (hereinafter referred to as "GBB") is pleased to announce today the completion of itsUSD 15M Pre-series B funding led by Tiger Jade Capital (hereinafter referred to as "Tiger Jade"). The funding was oversubscribed and upsized with participat...
EDGC (Eone Diagnomics Genome Center) recruits Sam Martin, Vice President of Overseas Business Development... to "accelerate entering the overseas market"
* An expert from Invitae and Ambry Genetics, U.S. genome analysis companies * EDGC states, "We will expand the overseas genome analysis market and advance into the global liquid biopsy technology market by recruiting a global talent in the genome field" * Worked in a non-profit research orga...
I-Mab Announces Approval from China CDE to Initiate Phase 3 Registrational Study of Lemzoparlimab in Combination with Azacitidine in Higher-Risk Myelodysplastic Syndrome
GAITHERSBURG, Md. and SHANGHAI, Sept, 13, 2022 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today announced that it has successfully completed an End-of-Phase 2 (...
Week's Top Stories
Most Reposted
Rocket Travel by Agoda Shares Revealing New Report and Showcases Solution to Transform Hotel Distribution
[Picked up by 313 media titles]
2024-11-21 10:30Rockwell Automation and Microsoft Deliver on a Shared Vision to Accelerate Industrial Transformation
[Picked up by 308 media titles]
2024-11-20 13:29Durabook and Parent Company, Twinhead International Corp., Celebrate 40 Years of Innovation in Computing Solutions
[Picked up by 301 media titles]
2024-11-20 16:30Travel loyalty programs to focus on offering personalized and flexible customer experiences in 2025
[Picked up by 298 media titles]
2024-11-19 10:42Philips and Edith Cowan University Australia Collaborate to Equip the Next Generation of Healthcare Professionals to leverage new technologies
[Picked up by 284 media titles]
2024-11-20 09:00