Biotechnology
Kintor Pharma Announces Completion of Subject Enrollment and Dosing in Phase I Clinical Trial of AR-PROTAC(GT20029) in China
SUZHOU, China, Aug. 9, 2022 /PRNewswire/ -- Kintor Pharmaceutical Limited ("Kintor Pharma", HKEX: 9939), a clinical-stage biotechnology company developing innovative small molecules and biological therapeutics, today announced that the company has completed the enrollment and dosing of 92 subjec...
Inmagene and HUTCHMED Announce First Participants in Global Phase I Trial of IMG-004
SAN DIEGO, SHANGHAI and HONG KONG, Aug. 9, 2022 /PRNewswire/ -- Inmagene
Biopharmaceutical ("Inmagene
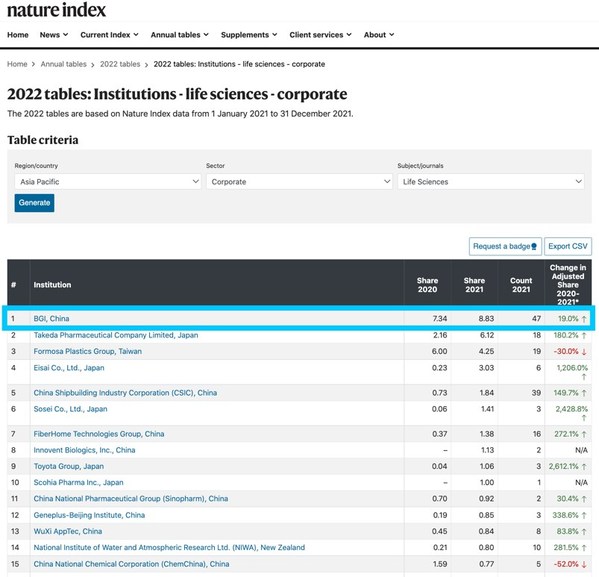
BGI Ranks No. 1 among APAC and China Life Science Corporations for Seven Consecutive Years: 2022 Nature Index Annual Tables Revealed
SHENZHEN, China, Aug. 8, 2022 /PRNewswire/ -- BGI has topped the Asia Pacific andChina life science corporate institution ranking table for the seventh year running, released in the 2022 Nature Index Annual Tables. With a 19 percent increase in its adjusted share metric, BGI ranked eighth among g...
ImmVira's Brand New oHSV Product MVR-C5252 Targeting Malignant Glioma Obtained FDA's Orphan Drug Designation
SHENZHEN, China, Aug. 7, 2022 /PRNewswire/ -- On August 1, 2022, U.S. time,
ImmVira's brand new oncolytic herpes simplex virus ("oHSV") product MVR-C5252
targeting Malignant Glioma has obtained Orphan Drug Designation ("ODD") from
U.S. Food and Drug Administration ("FDA").
Epigenic Therapeutics Raises $20 Million in Series Angel and Pre-A Funding to Advance Next Generation Gene Editing Therapy
SHANGHAI, Aug. 6, 2022 /PRNewswire/ -- Epigenic Therapeutics Co., Ltd., a frontier biotechnology company dedicated to developing next generation gene editing therapy utilizing regulation of epigenetic genome for wide variety of diseases, today announced it has secured$20 million in Series Angel a...
United BioPharma's Study Reveals New Class of Monoclonal Antibody for Effective Relief of Urticaria Symptoms
New results demonstrate a newer class of anti-IgE antibody, UB-221, with superior neutralization, synthesis reduction, and durable relief in targeting allergic diseases TAIPEI, Aug. 5, 2022 /PRNewswire/ -- United BioPharma (UBP) today announced their latest results featuring unprecedented and u...
Yiling's Lianhua Health Products Well-received: CICPE
SHIJIAZHUANG, China, Aug. 5, 2022 /PRNewswire/ -- As China's first national-level consumer expo and the largest such kind in theAsia-pacific region, the 2nd China International Consumer Products Expo (CICPE) attracted many audiences from different countries. And as the TOP 10 Chinese TCM Enterpr...
CGBio Signing a North America Out-license Contract on "Novosis rhBMP-2," a Bone Substitute with Orthofix, USA
* Embarking on a successful journey of exporting its home-grown product technology toNorth America, the largest market for bone substitutes * Enabling fast bone formation by applying bone morphogenetic protein (rhBMP-2) and next-generation synthetic carrier SEOUL, South Korea and LEWISVILLE, ...
RADIOPHARM ENTERS INTO STRATEGIC COLLABORATION WITH LANTHEUS AND ASSUMES PD-L1 LICENSING AGREEMENT FROM NANOMAB
* Radiopharm will shortly initiate a PD-L1 Phase 1 therapeutic study in Australia in patients with NSCLC * Radiopharm acquired from NanoMab, Ltd. worldwide rights to PD-L1 technology for therapeutic use, as well as to imaging rights inChina MELBOURNE, Australia, Aug. 3, 2022 /PRNewswire/ -- R...
ST Pharm Presents Phase 1 Clinical Trial Results of HIV Treatment Candidate at AIDS 2022
- STP0404 is the only clinical safety proven HIV treatment candidate with a novel mechanism that can block HIV re-activation. - STP0404 is expected to enter Phase 2a clinical trial in the US in 4Q 2022. SEOUL, South Korea, Aug. 3, 2022 /PRNewswire/ -- ST Pharm Co., Ltd. (237690:KOSDAQ) annou...
Kintor Pharma Announces Completion of Patient Enrollment in Phase II Clinical Trial of KX-826 for Treatment of Androgenetic Alopecia in the US
SUZHOU, China, Aug. 3, 2022 /PRNewswire/ -- Kintor Pharmaceutical Limited ("Kintor Pharma", HKEX: 9939), a clinical-stage biotechnology company developing innovative small molecules and biological therapeutics, today announced that the company completed the enrollment of 121 patients for its pha...
Sanyou Biopharmaceuticals Closed the Series B+ Financing Round of Tens of Millions of RMB Led by Huatai Zijin
SHANGHAI, Aug. 2, 2022 /PRNewswire/ -- Sanyou Biopharmaceuticals Co., Ltd. announced that it has closed the Series B+ financing round of tens of millions of RMB. In this round of financing led by Zijin Hongyun Fund and Huatai Guoxin Fund under Huatai Zijin, Sanyou also received follow-on investme...
GenKOre develops hypercompact base editing system
DAEJEON, South Korea , Aug. 2, 2022 /PRNewswire/ -- GenKOre (homepage:
www.genkore.com
Gracell Biotechnologies to Report Second Quarter 2022 Financial on Monday, August 15, 2022
SAN DIEGO, Calif., and SUZHOU and SHANGHAI, China, Aug. 2, 2022 /PRNewswire/ -- Gracell Biotechnologies Inc. (NASDAQ: GRCL) ("Gracell"), a global clinical-stage biopharmaceutical company dedicated to developing highly efficacious and affordable cell therapies for the treatment of cancer, today a...
CARsgen Appoints Dr. Hua Jiang as Executive Director
SHANGHAI, Aug. 2, 2022 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, announces thatDr. Hua Jiang ("Dr. Jiang") has been appointed as Executive Direct...
"OpenDEL®3.0 - DRUG DISCOVERY PARTNER" webinar offered by HitGen and Endpoints News coming up on Thursday 4th August
CHENGDU, China, Aug. 1, 2022 /PRNewswire/ -- "OpenDEL®3.0 – DRUG DISCOVERY PARTNER" webinar will be jointly held by HitGen Inc. and Endpoints News on Thursday 4th August, to present you with a high-quality DEL product with all DEL information sharing and technical support. Dr. Guansai Liu, Execut...
Yunji's Technology-empowered Private Label SUYE Celebrates Its 12th Anniversary
HANGZHOU, China, Aug. 1, 2022 /PRNewswire/ -- Yunji Inc. ("Yunji" or the "Company") (NASDAQ: YJ), a leading membership-based social e-commerce platform, today announced the celebration of its private-label skin care brand SUYE's 12th anniversary. SUYE generated over21 million RMB sales of its hig...
GreenLight Biosciences and Samsung Biologics complete first commercial-scale engineering run for mRNA Covid-19 vaccine
* GreenLight's messenger RNA production process is transferable to large-scale equipment and CMO facilities * Technology transfer and scale-up from lab bench to Samsung's commercial facility was completed in seven months * GreenLight's mRNA synthesis reaction had a titer of 12g/L at a commer...
Gracell Biotechnologies Appoints Accomplished Clinical Leader Dr. Wendy Li as Chief Medical Officer
Dr. Li brings deep oncology clinical development, medical affairs and U.S. regulatory experience SAN DIEGO, Calif. and SUZHOU and SHANGHAI, China, Aug. 1, 2022 /PRNewswire/ -- Gracell Biotechnologies Inc. ("Gracell" or the "Company", NASDAQ: GRCL), a global clinical-stage biopharmaceutical compa...
Eccogene Announces US IND Approval for THRβ agonist ECC4703
SHANGHAI, Aug. 1, 2022 /PRNewswire/ -- Eccogene announced that the U.S. Food and Drug Administration (FDA) has approved the investigational new drug (IND) application to commence Phase I trial of its thyroid hormone receptor agonist ECC4703 in U.S. This study will evaluate the safety, tolerabilit...
Week's Top Stories
Most Reposted
Rocket Travel by Agoda Shares Revealing New Report and Showcases Solution to Transform Hotel Distribution
[Picked up by 313 media titles]
2024-11-21 10:30Rockwell Automation and Microsoft Deliver on a Shared Vision to Accelerate Industrial Transformation
[Picked up by 308 media titles]
2024-11-20 13:29Durabook and Parent Company, Twinhead International Corp., Celebrate 40 Years of Innovation in Computing Solutions
[Picked up by 301 media titles]
2024-11-20 16:30Travel loyalty programs to focus on offering personalized and flexible customer experiences in 2025
[Picked up by 298 media titles]
2024-11-19 10:42Philips and Edith Cowan University Australia Collaborate to Equip the Next Generation of Healthcare Professionals to leverage new technologies
[Picked up by 284 media titles]
2024-11-20 09:00