Biotechnology
WuXi Biologics Extends Capabilities to Include Development and cGMP Manufacturing for Microbial-Derived Products
* WuXi Biologics expands its extensive integrated CRDMO services by offering development and cGMP manufacturing for microbial-derived products. HANGZHOU, China, July 4, 2022 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a global leading Contract Research, Development and Manufacturing ...
Arctic Vision Announces First Patient Enrolled in Phase III Clinical Trial of ARVN003 for Presbyopia
This is the first clinical trial approved in China for presbyopia drugs and Arctic Vision's study marks the first patient enrollment in a Phase III clinical trial for presbyopia drugs inChina. ARVN003 is expected to be the first approved drug for presbyopia inChina. SHANGHAI, July 4, 2022 /PRNew...
Abbisko Therapeutics Completed Dosing of First Patient for Its First-in-Class Highly Selective FGFR2/3 Inhibitor ABSK061
SHANGHAI, July 4, 2022 /PRNewswire/ -- Abbisko Therapeutics Co., Ltd. (HKEX Stock Code: 2256.HK, referred to "Abbisko Therapeutics" hereafter) today announced completion of dosing of the first patient in the Phase 1 clinical trial in advanced solid tumors for ABSK061, which becomes the first high...
ReviR Therapeutics Appoints Paul August, Ph.D., as Chief Scientific Officer
SOUTH SAN FRANCISCO, Calif., July 1, 2022 /PRNewswire/ -- ReviR Therapeutics, a biotechnology company focused on developing RNA-targeting small molecule drugs, announced the appointment ofPaul August, Ph.D., as the Chief Scientific Officer. "We are thrilled to welcome Dr. August to our team," s...
Ascentage Pharma Announces IND Clearance by the US FDA for First-in-Human Study of Novel EED Inhibitor APG-5918
SUZHOU, China and ROCKVILLE, Md., June 29, 2022 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that its novel inhibitor of the embryonic ectoderm...
iNtRON to Seek a New Clinical Partner for SAL200 Endolysin
* TONABACASE, the first registered INN by WHO for Endolysin * First-in-Class Endolysin-based Drug against Superbugs * US FDA Phase 2 IND Approval for TONABACASE, SAL200 * Roivant has returned the rights for the asset just before the first patient injection BOSTON and SEOUL, South Korea, Jun...
Congratulations to Dragon Boat Biopharmaceutical from Sanyou Biopharmaceuticals on the NMPA acceptance of the CLDN 18.2/CD47 bsAb clinical trial application
SHANGHAI, June 25, 2022 /PRNewswire/ -- On June 15, 2022, Dragon Boat announced that its IND application of the innovative anti-CLDN 18.2/CD47 bi-specific antibody (bsAb) injection (R&D code: BC007) was officially accepted by National Medical Products Administration (NMPA) under the acceptance nu...
Sanyou Biopharmaceuticals forged strategic partnership with Dragon Sail Pharmaceutical to upgrade integrated innovative antibody drug R&D
SHANGHAI, June 24, 2022 /PRNewswire/ -- Sanyou Biopharmaceuticals, a biological high-tech enterprise focusing on R&D and services of innovative antibody drugs, and Dragon Sail Pharmaceutical, aCDMO and CMO enterprise dedicated to providing world-leading high-end biological drug manufacture servic...
Daewoong Pharmaceutical begins multinational phase 2 clinical trial for DWN12088, a new drug for idiopathic pulmonary fibrosis
- U.S. Food and Drug Administration (FDA) approved the IND for the phase 2 clinical trial for patients with idiopathic pulmonary fibrosis - Daewoong Pharmaceutical to start a multinational phase 2 clinical trial for DWN12088 in September SEOUL, South Korea, June 24, 2022 /PRNewswire/ -- Daewoong...
Alterity Therapeutics Announces Regulatory Authorization to Proceed with ATH434 Phase 2 Clinical Trial in Italy
MELBOURNE, Australia and SAN FRANCISCO, June 23, 2022 /PRNewswire/ -- Alterity Therapeutics (ASX: ATH, NASDAQ: ATHE) ("Alterity" or "the Company"), a biotechnology company dedicated to developing disease modifying treatments for neurodegenerative diseases, today announced that the Italian Medicin...
Viva Biotech Successfully Held The 3rd Annual Partnership Summit
SHANGHAI, June 23, 2022 /PRNewswire/ -- June 16th-20th, 2022 (Beijing time), Viva Biotech 2022 Partnership Summit was successfully held. Over 300 attendees joined the Summit, including founders from portfolio companies, representatives from global investment institutions, R&D heads from pharmaceu...
I-Mab Receives Top Rankings in Five Categories by Institutional Investor
GAITHERSBURG, Md. and SHANGHAI, June 23, 2022 /PRNewswire/ -- I-Mab ("I-Mab" or the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today announced that it was ranked among the top companies i...
Mojia Biotech Completes Series B Financing to Advance Manufacturing of Bio-based Materials
SHANGHAI, June 22, 2022 /PRNewswire/ -- Mojia Biotech, a Shanghai-based leading bio-manufacturing company dedicated to sustainable development and carbon neutrality, announced the completion of an $80 million Series B Financing. The funds will be used to commercialize its Viridimin™ brand of anim...
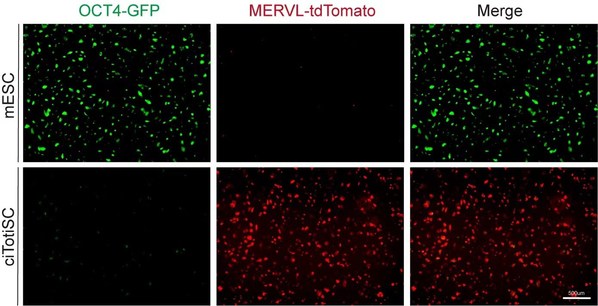
Scientists take the first step to master an all-powerful cell type in the beginning of life
Sheng Ding and his team at Tsinghua University School of Pharmaceutical Sciences publish innovative work in Nature BEIJING, June 21, 2022 /PRNewswire/ -- From cloning to regeneration, how to find alternative paths to create or rejuvenate life has been one of the big questions for biologists. It ...
Acepodia Announces FDA Clearance of IND Application for ACE1831, an Anti-CD20 Armed Allogeneic gamma delta T-cell Therapy Candidate to Treat Patients with non-Hodgkin's Lymphoma
ACE1831 is a potential antibody‑armed allogeneic gamma delta T cell therapy developed using Acepodia's unique antibody-cell conjugation (ACC) technology as an optimized T cell engager platform to treat patients with non-Hodgkin's lymphoma ALAMEDA, Calif. and TAIPEI, Taiwan, June 20, 2022 /PRNews...
2022 Tang Prize in Biopharmaceutical Science Honors Three Scientists for Developing COVID-19 mRNA Vaccines
TAIPEI, Taiwan , June 18, 2022 /PRNewswire/ -- After the 2014 and 2016 winners for the Tang Prize in Biopharmaceutical Sciences were crowned the Nobel Prize in 2018 and 2020 respectively, this category has continued to garner much attention worldwide. After much waiting, names of the latest winne...
2022 Tang Prize in Biopharmaceutical Science Honors Three Scientists for Developing COVID-19 mRNA Vaccines
TAIPEI, Taiwan, June 18, 2022 /PRNewswire/ -- After the 2014 and 2016 winners for the Tang Prize in Biopharmaceutical Sciences were crowned the Nobel Prize in 2018 and 2020 respectively, this category has continued to garner much attention worldwide. After much waiting, names of the latest winner...
US FDA AWARDS ORPHAN DRUG DESIGNATION (ODD) TO PAXALISIB FOR AT/RT, A RARE FORM OF CHILDHOOD BRAIN CANCER
SYDNEY, June 17, 2022 /PRNewswire/ -- Kazia Therapeutics Limited (NASDAQ: KZIA; ASX: KZA), an oncology-focused drug development company, is pleased to announce that the United States Food and Drug Administration (FDA) has awarded Orphan Drug Designation (ODD) to Kazia's paxalisib for the treatmen...
WuXi ATU and Wugen Announce Manufacturing Partnership to Expedite the Delivery of Novel Cell Cancer Immunotherapies
PHILADELPHIA and ST. LOUIS, MO and SAN DIEGO, June 15, 2022 /PRNewswire/ -- WuXi Advanced Therapies (WuXi ATU), a global Contract Testing, Development and Manufacturing Organization (CTDMO), and Wugen Inc., a clinical-stage biotechnology company based inSt. Louis and San Diego, today announced a ...
I-Mab Partner MorphoSys Announces New License Agreements for Felzartamab and TJ210
SHANGHAI and GAITHERSBURG, Md., June 15, 2022 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today announced two assets the Company has licensed from partner Morpho...
Week's Top Stories
Most Reposted
Rocket Travel by Agoda Shares Revealing New Report and Showcases Solution to Transform Hotel Distribution
[Picked up by 313 media titles]
2024-11-21 10:30Rockwell Automation and Microsoft Deliver on a Shared Vision to Accelerate Industrial Transformation
[Picked up by 308 media titles]
2024-11-20 13:29Durabook and Parent Company, Twinhead International Corp., Celebrate 40 Years of Innovation in Computing Solutions
[Picked up by 301 media titles]
2024-11-20 16:30Travel loyalty programs to focus on offering personalized and flexible customer experiences in 2025
[Picked up by 298 media titles]
2024-11-19 10:42Philips and Edith Cowan University Australia Collaborate to Equip the Next Generation of Healthcare Professionals to leverage new technologies
[Picked up by 284 media titles]
2024-11-20 09:00