Health Care/Hospital
Akeso's IL-17A MONOCLONAL ANTIBODY (GUMOKIMAB) COMPLETION OF PATIENT ENROLLMENT IN PHASE II CLINICAL TRIAL FOR THE TREATMENT OF ANKYLOSING SPONDYLITIS
HONG KONG, Dec. 27, 2021 /PRNewswire/ -- Today, Akeso, Inc. (09926.HK) announces that Gumokimab (IL-17A monoclonal antibody, AK111), an innovative drug independently developed by the Company for the treatment of active ankylosing spondylitis has been completed. Such clinical trial aims to evalua...
Regor Therapeutics Announces U.S. FDA Authorization to Conduct Regor's First-in-Human Clinical Trial with the Next Generation Targeted Inhibitor RGT-419B for Oncology
SHANGHAI, Dec. 27, 2021 /PRNewswire/ -- On December 23, Regor Therapeutics, a clinical-stage biotech company,announced authorization from the US Food and Drug Administration (FDA) to proceed with Regor's Phase 1 clinical development plans for RGT-419B. RGT-419B is a new generation CDK2/4/6, smal...
I-Mab Announces IND Approval from China NMPA for Phase 2 Clinical Trial of Enoblituzumab in Combination with Pembrolizumab in Solid Tumors
SHANGHAI and GAITHERSBURG, Md., Dec. 27, 2021 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, announced that the Center for Drug Evaluation (CDE) ofChina's National ...
Kintor Pharma Provides Update on One of its Three Multi-Regional Phase 3 Trials of Proxalutamide for COVID-19
SUZHOU, China, Dec. 27, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited ("Kintor Pharma," HKEX: 9939), a clinical-stage biotechnology company developing innovative small molecule and biological therapeutics, today provided an update on its multi-regional study of proxalutamide for the treatme...
Laekna Presents Vision and Strategy at NewYorkBIO/NYSE Emerging Company Showcase
SHANGHAI and WARREN, N.J., Dec. 24, 2021 /PRNewswire/ -- Laekna was invited by NewYorkBIO and the New York Stock Exchange (NYSE) to present, as an emerging biotech company at the "Emerging Company Showcase" that was held earlier this month inNew York. Dr. Guy Rosenthal, Vice President, Head of C...
Curocell, Breaks Ground on 'CAR-T Manufacturing GMP Facility'
DAEJEON, South Korea, Dec. 23, 2021 Curocell, currently on PhaseⅠCAR-T Therapy
clinical trial withCRC01 (anbalcabtagene autoleucel) has recently broken ground
for the new CAR-T Center in Dungok Residential & Industrial Area in Daejeon
International Science & Business Belt.
Akeso's PCSK9 MONOCLONAL ANTIBODY (EBRONUCIMAB) EARLY COMPLETION OF PATIENT ENROLLMENT IN A PHASE III CLINICAL TRIAL FOR PRIMARY HYPERCHOLESTEROLEMIA AND MIXED HYPERLIPIDEMIA
HONG KONG, Dec. 22, 2021 /PRNewswire/ -- Today, Akeso, Inc. (09926.HK) announces that Ebronucimab (PCSK9 monoclonal antibody, research and development code: AK102), jointly developed by the Company and Dawnrays Biotechnology Capital (Asia) Ltd., completed patient enrollment early in a pivotal re...
Yiling Pharmaceutical's Patent Depression-resolving Drug Approved for Marketing in China
BEIJING, Dec. 22, 2021 /PRNewswire/ -- According to Yiling Pharmaceutical's notice onThursday (UTC+8), the Depression-resolving and Restlessness-relieving Capsules have been approved for marketing via the obtained Drug Registration Certificate issued byChina's National Medical Products Administra...
Aerogen Pharma Enters into Exclusive Agreements with Nuance Pharma to Advance Treatment of Respiratory Distress Syndrome in Premature Infants in China
SHANGHAI, Dec. 22, 2021 /PRNewswire/ -- Aerogen Pharma, a developer of innovative inhaled treatments for patients in critical care, and Nuance Pharma ("Nuance"), a specialty care focused biopharma with late-stage clinical programs and existing commercial operations, today announce an exclusive a...
Deargen Annonuces Patent Application for a TNBC Target Novel Drug Candidate Using AI
* Using a self-developed AI platform, it takes only 10 weeks from the discovery of the anticancer drug targets to the discovery and synthesis of new compounds. * Proven capabilities for NCE compound discovery and design, and the establishment of a R&D center(iDear Center) to continuously deve...
AMO Pharma Announces Expansion of Pivotal REACH-CDM Study in Congenital Myotonic Dystrophy
- Additional research centers now enrolling patients in Australia and New Zealand - Company has achieved 50 percent of target enrollment in global Phase 3 trial LONDON, Dec. 23, 2021 /PRNewswire/ -- AMO Pharma Limited ("AMO Pharma"), a privately held biopharmaceutical company focusing on r...
Exopharm Gains US Patent Enabling Commercial Production of Exosome Medicines
* US Patent and Trademark Office has granted Exopharm patent US 11202805, for its LEAP™ exosome purification technology. * LEAP™ enables large-scale, clinical grade commercial production of exosomes needed to underpin the emerging field of exosome medicines. * Exopharm is seeking potential p...
TCM provides new alternatives for the fight against COVID-19
BEIJING, Dec. 21, 2021 /PRNewswire/ -- A news report by haiwainet.cn: During the 18th World Congress of Traditional Chinese Medicine held on4 December 2021, experts fromSpain, France, Australia and other countries and regions highly recognized the role of Traditional Chinese Medicine (TCM) in fig...
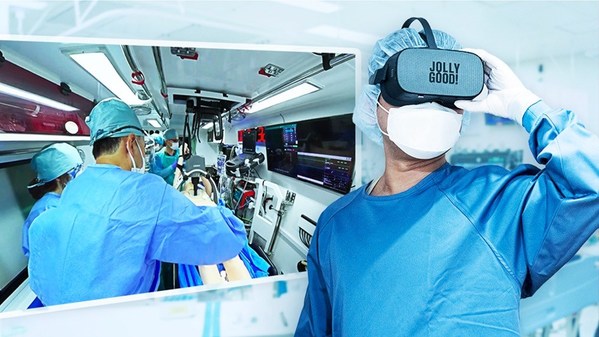
Jolly Good develops a medical VR filming system exclusively for ambulances with physicians
TOKYO, Dec. 21, 2021 /PRNewswire/ -- Jolly Good Inc. ("Jolly Good") developed a medical VR filming system for ambulances with physicians in collaboration with Nippon Medical School Hospital Emergency & Critical Care Medicine (Bunkyo-ku, Tokyo; Director: Shoji Yokobori). Together, they will begin t...
Hanjiao Group Invited by Rising China Interview Show
BEIJING, Dec. 21, 2021 /PRNewswire/ -- Hanjiao Group, Inc. ("Hanjiao" or the "Company") (OTC Pink: HJGP), a Nevada holding corporation that, through its variable interest entity, is engaged in providing home care services and related healthcare products to the middle-aged and elderly communities ...
Henlius' anti-PD-1 mAb MRCT achieved 15.38 months OS in first-line treatment of SCLC, reducing the risk of death by 38% of the overall population
* The ASTRUM-005 states that serplulimab combined with carboplatin-etoposide prolonged median OS in both the overall population and the Asian subgroup, the median overall survival (OS) in the serplulimab and placebo groups were 15.38 and 11.10 months, respectively, reducing risk of death by 38%...
I-Mab Strengthens Management Team Designed to Accelerate Global Pipeline Development and Transformation Towards Commercialization
- New appointments as part of the Company's strategic development plans to accelerate transformation towards commercialization - Dr. Andrew Zhu, an internationally renowned oncologist, appointed as President and board director to lead the Company's R&D organization, focusing on global pipelin...
First Patient Dosed in Australia in ATG-101 First-in-Human Trial
SHANGHAI AND HONG KONG, Dec. 19, 2021 /PRNewswire/ -- Antengene Corporation Limited ("Antengene", SEHK: 6996.HK), a leading innovative global biopharmaceutical company dedicated to discovering, developing and commercializing first-in-class and/or best-in-class therapeutics in hematology and onco...
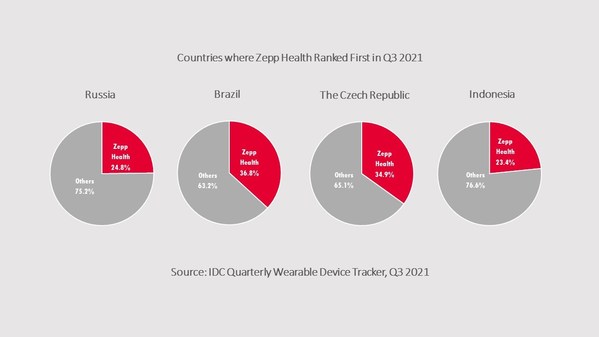
ZEPP HEALTH REPORTS SURGE IN GLOBAL SHIPMENTS IN Q3 2021
CUPERTINO, Calif., Dec. 17, 2021 /PRNewswire/ -- Zepp Health (NYSE: ZEPP) continued its robust growth in global adult watch shipments in the third quarter of 2021, according to the Q3 2021 Quarterly Wearable Device Tracker by the International Data Corporation (IDC) - a leading market intelligenc...
Senhwa's Silmitasertib Receives US FDA Orphan Drug Designation for the Treatment of Medulloblastoma
TAIPEI and SAN DIEGO, Dec. 17, 2021 /PRNewswire/ -- Senhwa Biosciences, Inc. (TPEx: 6492), a drug development company focusing on first-in-class therapeutics for oncology, rare diseases, and novel coronaviruses, announced that the US Food and Drug Administration (FDA) has granted Orphan Drug Des...
Week's Top Stories
Most Reposted
Troy Animal Healthcare Strengthens Quality Operations with Veeva Vault Quality
[Picked up by 321 media titles]
2024-05-17 07:00Ultima Markets Recognised as Most Popular Broker 2024 by BrokersView
[Picked up by 319 media titles]
2024-05-15 12:00Home to half of the world's top 10 trending tourism destinations, Asia Pacific is making a comeback: Mastercard Economics Institute on travel in 2024
[Picked up by 303 media titles]
2024-05-16 15:00US-BASED PANDUIT ELEVATES MANUFACTURING LANDSCAPE WITH NEW STATE-OF-THE-ART PLANT IN JOHOR BAHRU, MALAYSIA
[Picked up by 274 media titles]
2024-05-16 09:39Trip.com Group and Rezdy Join Forces to Offer New Travel Experiences Around the World
[Picked up by 229 media titles]
2024-05-14 18:07