Health Care/Hospital
Harbour BioMed Announces IND Approval for B7H4x4-1BB Bispecific Antibody
CAMBRIDGE, Mass. and ROTTERDAM, Netherlands and SUZHOU, China, June 8, 2022 /PRNewswire/ --Harbour BioMed ("HBM", HKEX: 02142) announced that China National Medical Products Administration (NMPA) had approved the investigational new drug (IND) application to commence phase I trial of its B7H4x4-...
Waterdrop Inc. to Report First Quarter 2022 Financial Results on June 15, 2022
BEIJING, June 8, 2022 /PRNewswire/ -- Waterdrop Inc. (NYSE: WDH) ("Waterdrop" or the "Company"), a leading technology platform dedicated to insurance and healthcare service with a positive social impact, today announced that it will report its unaudited financial results for the first quarter end...
ASCO 2022 | Ascentage Pharma Releases for the First Time Results of its FAK/ALK/ROS1 inhibitor APG-2449 Demonstrating Safety and Efficacy in Patients with Advanced NSCLC
SUZHOU, China, and ROCKVILLE, MD, June 7, 2022 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that it has released the results from its Phase I ...
ASCO 2022 | Ascentage Pharma Releases Updated Data Demonstrating Lisaftoclax's (APG-2575) Therapeutic Potential in Patients with R/R CLL/SLL
SUZHOU, China and ROCKVILLE, Md., June 7, 2022 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that it has released the updated results from a Pha...
Snap Fitness Announces "Earn Your Apple Watch" Program
New Incentive Program Rewards Active Members while Benefiting Franchisees with Brand Differentiation CHANHASSEN, Minn., June 8, 2022 /PRNewswire/ -- In a strategic move that further sets them apart as a leader in the fitness space, Snap Fitness announced today the global launch of the "Earn Y...
The 3rd BIO International Convention 2022 is Approaching
SAN DIEGO, June 7, 2022 /PRNewswire/ -- With the 3rd BIO International Convention 2022 soon approaching, Neurophth Therapeutics is looking forward to discussing collaboration opportunities in gene therapy for both rare and common ocular diseases. Neurophth Therapeutics is a leading clinical-stag...
PHASE II STUDY OF PAXALISIB IN BRAIN METASTASES ADVANCES TO EXPANSION STAGE IN BREAST CANCER BRAIN METASTASES COHORT
SYDNEY, June 7, 2022 Kazia Therapeutics Limited (NASDAQ: KZIA; ASX: KZA), an oncology-focused drug development company, is pleased to announce that a phase II, genomically-guided study of multiple therapies in patients with brain metastases, led by the Alliance for Clinical Trials in Oncology (NC...
Chordia Therapeutics Announces Interim Results of the Phase 1 Clinical Trial of CLK Inhibitor CTX-712 at the 2022 ASCO Annual Meeting
KANAGAWA, Japan, June 7, 2022 /PRNewswire/ -- Chordia Therapeutics Inc. ("Chordia"), a biotech company engaged in the research and development of novel therapies for cancers, today announced that it has presented the interim results from the Phase 1 clinical trial of CTX-712, a selective pan-CDC-...
ASCO 2022 | Ascentage Pharma Releases Updated Results Demonstrating the Therapeutic Potential of Alrizomadlin (APG-115) plus Pembrolizumab in Patients with Solid Tumors who Progressed on Immunotherapies
SUZHOU, China, and ROCKVILLE, Md., June 6, 2022 /PRNewswire/ -- scentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that it has released the updated results from a Pha...
ASCO 2022 | The First Dataset of Olverembatinib (HQP1351) in Patients with GIST Demonstrates Therapeutic Potential with a Clinical Benefit Rate of 83.3%
SUZHOU, China, and ROCKVILLE, Md., June 6, 2022 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that it has released the latest results from a Pha...
iNtRON Completes GLP-TOX Studies of BAL200
* A novel drug candidate for anthrax received orphan drug designation (ODD) by the US FDA. BOSTON and SEOUL, Korea, June 6, 2022 /PRNewswire/ -- iNtRON Biotechnology ("iNtRON" or "Company") announced today that the company has successfully completed the GLP toxicology studies of BAL200. The co...
MGI Announces Commercial Availability of DNBSEQ™ Sequencers* in the United States
SAN JOSE, Calif., June 6, 2022 /PRNewswire/ -- MGI Americas (MGI), today announced that its innovative CoolMPS sequencing chemistry and instruments* will become commercially available inthe United States beginning from August 29,2022. More details about the launch will be revealed at the 22nd ...
IND approval from the US FDA for Phase II SAR-Bombesin imaging trial in prostate cancer
Highlights * IND approval received for SAR-Bombesin product, enabling a Phase II "SABRE" imaging trial to detect prostate cancer in up to 50 PSMA-negative participants in the US * Approximately 20% of prostate cancer patients with biochemical recurrence (BCR) are PSMA-PET negative and theref...
Senhwa's Pindnarulex in Combination Study with Pfizer's Talazoparib for the Treatment of Prostate Cancer Granted Approval to Initiate from Australian HREC
TAIPEI and SAN DIEGO, June 6, 2022 /PRNewswire/ -- Senhwa Biosciences, Inc. (TPEx: 6492), a drug development company focusing on first-in-class therapeutics for oncology, rare diseases, and novel coronaviruses, announced that it has received written approval from the Human Research Ethics Committ...
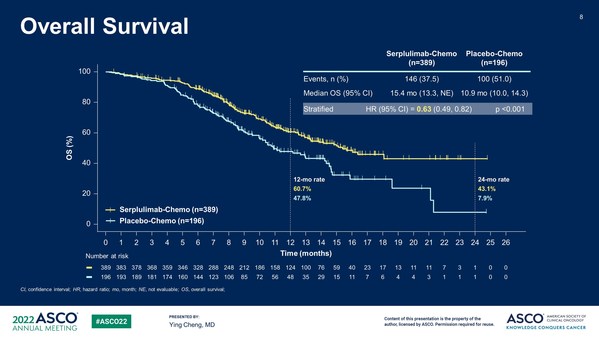
ASTRUM-005: Henlius Released Phase 3 Study Results for the First-line Treatment of Small Cell Lung Cancer of Serplulimab at ASCO 2022
SHANGHAI, June 6, 2022 /PRNewswire/ -- Shanghai Henlius Biotech, Inc. (2696.HK) announced that an international randomized phase 3 study (ASTRUM-005) of HANSIZHUANG (serplulimab), an anti-PD-1 mAb independently developed by Henlius, as first-line treatment for extensive-stage small-cell lung can...
CARsgen Therapeutics Presents Updated Data for CT041 Claudin18.2 CAR T-cells in Solid Tumors at ASCO 2022
SHANGHAI, June 6, 2022 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, announces that at the 2022 American Society of Clinical Oncology (ASCO) Annual M...
CStone and Pfizer announce NMPA approval of sugemalimab in patients with unresectable stage III non-small cell lung cancer
* The National Medical Products Administration approved sugemalimab for the treatment of patients with unresectable stage III non-small cell lung cancer whose disease has not progressed following concurrent or sequential platinum-based chemoradiotherapy * Sugemalimab became the first anti-PD-...
OriCiro Announces Series B2 Financing to Advance Cell-Free DNA Technology for Innovative Therapeutics and Synthetic Biology
TOKYO, June 5, 2022 /PRNewswire/ -- OriCiro Genomics, a pioneer in cell-free synthesis and amplification of genome-scale large DNA for advanced therapy and synthetic biology, today announced that it has closed Series B2 financing from Asahi Kasei Medical Co., Ltd. OriCiro has advanced its busine...
Akeso releases promising data of Ivonescimab (PD-1/VEGF BsAbs, AK112) for advanced NSCLC at ASCO 2022
HONG KONG, June 5, 2022 /PRNewswire/ -- Akeso, Inc. (9926.HK) ( "Akeso" ), a China-based biopharmaceutical company focusing on the development and commercialization of innovative therapeutic antibodies for Oncology & Immunology, released clinical details in poster presentation featuring phase Ib...
Akeso announces oral presentation featuring promising clinical data of Cadonilimab (PD-1/CTLA-4 BsAbs, AK104) for the first-line treatment of R/M cervical cancer at ASCO 2022
HONG KONG, June 5, 2022 /PRNewswire/ -- Akeso, Inc. (9926.HK) ( "Akeso" ), a China-based biopharmaceutical company focusing on the development and commercialization of innovative therapeutic antibodies for Oncology & Immunology, released updated results of Cadonilimab (PD-1/CTLA-4 Bispecific, AK...
Week's Top Stories
Most Reposted
Rocket Travel by Agoda Shares Revealing New Report and Showcases Solution to Transform Hotel Distribution
[Picked up by 314 media titles]
2024-11-21 10:30QDX Co-Founder Giuseppe Barca Awarded Prestigious Gordon Bell Prize, Pioneering Advancements in Drug Discovery Technology
[Picked up by 299 media titles]
2024-11-26 18:49Southeast Asian Consumers Raise Online Security Concerns, New GSMA Survey Shows
[Picked up by 295 media titles]
2024-11-26 13:00TAILG's First Flagship Store in Indonesia Grandly Opens
[Picked up by 283 media titles]
2024-11-22 21:59MediSun Energy Raises $8.75M Seed Round with Vynn Capital to Drive MENA Expansion and Advance Osmotic Energy Innovation
[Picked up by 278 media titles]
2024-11-25 10:00