Health Care/Hospital
iNtRON Completes GLP-TOX Studies of BAL200
* A novel drug candidate for anthrax received orphan drug designation (ODD) by the US FDA. BOSTON and SEOUL, Korea, June 6, 2022 /PRNewswire/ -- iNtRON Biotechnology ("iNtRON" or "Company") announced today that the company has successfully completed the GLP toxicology studies of BAL200. The co...
MGI Announces Commercial Availability of DNBSEQ™ Sequencers* in the United States
SAN JOSE, Calif., June 6, 2022 /PRNewswire/ -- MGI Americas (MGI), today announced that its innovative CoolMPS sequencing chemistry and instruments* will become commercially available inthe United States beginning from August 29,2022. More details about the launch will be revealed at the 22nd ...
IND approval from the US FDA for Phase II SAR-Bombesin imaging trial in prostate cancer
Highlights * IND approval received for SAR-Bombesin product, enabling a Phase II "SABRE" imaging trial to detect prostate cancer in up to 50 PSMA-negative participants in the US * Approximately 20% of prostate cancer patients with biochemical recurrence (BCR) are PSMA-PET negative and theref...
Senhwa's Pindnarulex in Combination Study with Pfizer's Talazoparib for the Treatment of Prostate Cancer Granted Approval to Initiate from Australian HREC
TAIPEI and SAN DIEGO, June 6, 2022 /PRNewswire/ -- Senhwa Biosciences, Inc. (TPEx: 6492), a drug development company focusing on first-in-class therapeutics for oncology, rare diseases, and novel coronaviruses, announced that it has received written approval from the Human Research Ethics Committ...
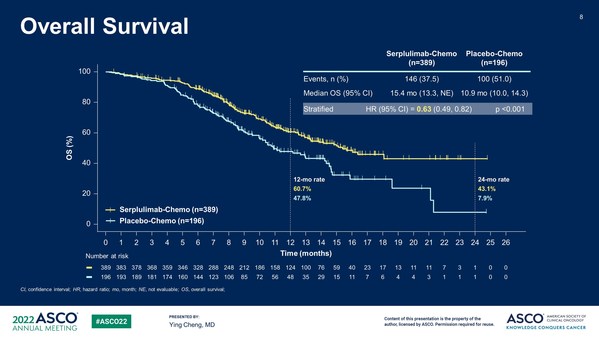
ASTRUM-005: Henlius Released Phase 3 Study Results for the First-line Treatment of Small Cell Lung Cancer of Serplulimab at ASCO 2022
SHANGHAI, June 6, 2022 /PRNewswire/ -- Shanghai Henlius Biotech, Inc. (2696.HK) announced that an international randomized phase 3 study (ASTRUM-005) of HANSIZHUANG (serplulimab), an anti-PD-1 mAb independently developed by Henlius, as first-line treatment for extensive-stage small-cell lung can...
CARsgen Therapeutics Presents Updated Data for CT041 Claudin18.2 CAR T-cells in Solid Tumors at ASCO 2022
SHANGHAI, June 6, 2022 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, announces that at the 2022 American Society of Clinical Oncology (ASCO) Annual M...
CStone and Pfizer announce NMPA approval of sugemalimab in patients with unresectable stage III non-small cell lung cancer
* The National Medical Products Administration approved sugemalimab for the treatment of patients with unresectable stage III non-small cell lung cancer whose disease has not progressed following concurrent or sequential platinum-based chemoradiotherapy * Sugemalimab became the first anti-PD-...
OriCiro Announces Series B2 Financing to Advance Cell-Free DNA Technology for Innovative Therapeutics and Synthetic Biology
TOKYO, June 5, 2022 /PRNewswire/ -- OriCiro Genomics, a pioneer in cell-free synthesis and amplification of genome-scale large DNA for advanced therapy and synthetic biology, today announced that it has closed Series B2 financing from Asahi Kasei Medical Co., Ltd. OriCiro has advanced its busine...
Akeso releases promising data of Ivonescimab (PD-1/VEGF BsAbs, AK112) for advanced NSCLC at ASCO 2022
HONG KONG, June 5, 2022 /PRNewswire/ -- Akeso, Inc. (9926.HK) ( "Akeso" ), a China-based biopharmaceutical company focusing on the development and commercialization of innovative therapeutic antibodies for Oncology & Immunology, released clinical details in poster presentation featuring phase Ib...
Akeso announces oral presentation featuring promising clinical data of Cadonilimab (PD-1/CTLA-4 BsAbs, AK104) for the first-line treatment of R/M cervical cancer at ASCO 2022
HONG KONG, June 5, 2022 /PRNewswire/ -- Akeso, Inc. (9926.HK) ( "Akeso" ), a China-based biopharmaceutical company focusing on the development and commercialization of innovative therapeutic antibodies for Oncology & Immunology, released updated results of Cadonilimab (PD-1/CTLA-4 Bispecific, AK...
Akeso releases promising data of Ivonescimab (PD-1/VEGF BsAbs, AK112) combined with chemotherapy in advanced NSCLC at ASCO 2022
HONG KONG, June 5, 2022 /PRNewswire/ -- Akeso, Inc. (9926.HK) ( "Akeso" ), a China-based biopharmaceutical company focusing on the development and commercialization of innovative therapeutic antibodies for Oncology & Immunology, released clinical details in poster discussion featuring a phase II ...
Dogness (International) Corporation Closes $12 Million Offering
DONGGUAN, China, June 3, 2022 /PRNewswire/ -- Dogness (International) Corporation ("Dogness" or the "Company") (NASDAQ: DOGZ), a developer and manufacturer of a comprehensive line of Dogness-branded, OEM and private label pet products, today announced that it closed a previously announced offerin...
Gracell Biotechnologies Schedules Clinical Update Call After EHA2022
SAN DIEGO, Calif. and SUZHOU and SHANGHAI, China, June. 3, 2022 /PRNewswire/ -- Gracell Biotechnologies Inc. (NASDAQ: GRCL) ("Gracell"), a global clinical-stage biopharmaceutical company dedicated to developing highly efficacious and affordable cell therapies for the treatment of cancer, today a...
ProSomnus® to Present Data at SLEEP 2022, the Annual Meeting of the American Academy of Sleep Medicine and the Sleep Research Society
SAN FRANCISCO, June 3, 2022 /PRNewswire/ -- ProSomnus, a leader in patient-preferred medical devices for the treatment of Obstructive Sleep Apnea (OSA), today announced the results of three studies evaluating its FDA-cleared Oral Appliance Therapy (OAT) devices in the treatment of OSA. ProSomnus ...
KAZIA PRESENTS POSITIVE FINAL DATA FROM PHASE II STUDY OF PAXALISIB IN NEWLY DIAGNOSED GLIOBLASTOMA AT ASCO CONFERENCE
SYDNEY, June 3, 2022 /PRNewswire/ -- Kazia Therapeutics Limited (NASDAQ: KZIA; ASX: KZA), an oncology-focused drug development company, is pleased to announce final data from its phase II study of paxalisib as first line therapy in patients with glioblastoma (NCT03522298). The data is the subj...
Sanyou Super-Trillion Innovative Antibody Drug Discovery Platform Announced - World Premiere
SHANGHAI, June 2, 2022 /PRNewswire/ -- Sanyou Biopharmaceuticals officially
launched STAL (Super-Trillion Antibody Libraries) onJune 3, 2022, which is a
super-trillion innovative antibody drug discovery platform of Sanyou's own
intellectual property.
Neurophth Therapeutics Appoints Xiaoning Guo as Chief Medical Officer
SHANGHAI and SAN DIEGO, June 2, 2022 /PRNewswire/ -- Neurophth Therapeutics, Inc., (hereinafter referred to as "Neurophth") today announced the appointment ofXiaoning Guo, Ph.D., as the Chief Medical Officer (CMO). Dr. Xiaoning Guo will have overall responsibility for the drug development functio...
ProfoundBio Announces Completion of $70 Million Series A+ Financing to Advance Antibody-Drug Conjugate (ADC) Programs into the Clinic
- Advance PRO1184 and PRO1160 into clinical trials - Accelerate multiple programs into preclinical development and further strengthen/expand innovative technology platforms - Build vertically integrated internal capabilities for more efficient drug development WOODINVILLE, Wash. and SUZHOU, China...
Alterity Therapeutics Launches ATH434 Phase 2 Clinical Trial for the Treatment of Patients with Multiple System Atrophy
Multiple System Atrophy is a rare, rapidly progressive, neurodegenerative disease that causes profound disability MELBOURNE, Australia, June 2, 2022 /PRNewswire/ -- Alterity Therapeutics (ASX: ATH, NASDAQ: ATHE) ("Alterity" or "the Company"), a biotechnology company dedicated to developing disea...
Beijing public transport to auto-check health code, NAT results
BEIJING, June 2, 2022 /PRNewswire/ -- This is a report from China.org.cn. Beijing subways and buses will implement a new ticketing system that attaches passengers' health codes to their transit cards, said the municipal transport authority on Thursday. According to the new protocol, passengers' ...
Week's Top Stories
Most Reposted
QDX Co-Founder Giuseppe Barca Awarded Prestigious Gordon Bell Prize, Pioneering Advancements in Drug Discovery Technology
[Picked up by 303 media titles]
2024-11-26 18:49ITAP 2024 sets the stage for a more connected advanced factory ecosystem with focus on AI, advanced robotics and sustainability
[Picked up by 296 media titles]
2024-11-28 17:24Southeast Asian Consumers Raise Online Security Concerns, New GSMA Survey Shows
[Picked up by 296 media titles]
2024-11-26 13:00MediSun Energy Raises $8.75M Seed Round with Vynn Capital to Drive MENA Expansion and Advance Osmotic Energy Innovation
[Picked up by 280 media titles]
2024-11-25 10:00Mentech at COP29: Showing the Eco-friendly Lifestyle with Technological Innovation
[Picked up by 275 media titles]
2024-11-29 20:33