Medical/Pharmaceuticals
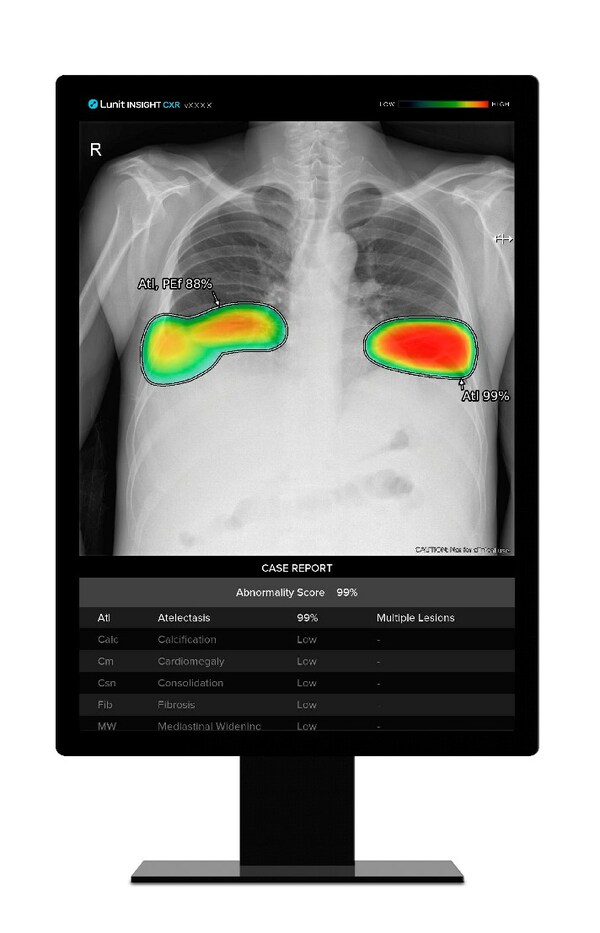
Lunit INSIGHT CXR Excels in Lung Nodule Detection - Exceptional Performance in Head-to-Head Study published in Radiology
- Lunit's AI-powered chest X-ray analysis solution shows highest AUC of 0.93 in a recent multi-center, multi-use case, multi-vendor lung nodule detection study SEOUL, South Korea, Jan. 17, 2024 /PRNewswire/ -- Lunit (KRX:328130.KQ), a leading provider of AI-powered solutions for cancer diagnostic...
Olverembatinib Included in Newest Guidelines on Chronic Myeloid Leukemia (CML) Management from the National Comprehensive Cancer Network (NCCN)
SUZHOU, China, and ROCKVILLE, Md., Jan. 16, 2024 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancer, chronic hepatitis B (CHB), and age-related diseases, announced today that olverembatinib (R&D Code: HQP1351) has been ...
WuXi Biologics Granted U.S. Patent for Proprietary Bispecific Antibody Technology Platform WuXiBody™
SHANGHAI, Jan. 16, 2024 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a leading global Contract Research, Development and Manufacturing Organization (CRDMO), today announced that a patent for WuXiBodyTM – a proprietary, highly flexible engineering platform that greatly enhances the deve...
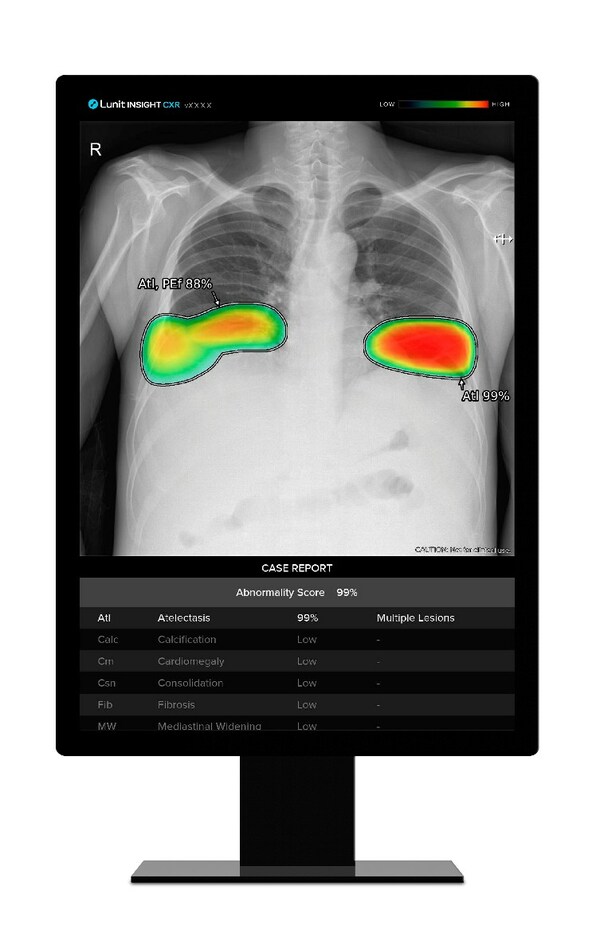
Lunit AI Solutions to Power Samsung's X-ray Devices for Advanced Chest Screening
* Three-year supply contract: Lunit INSIGHT CXR and Lunit INSIGHT CXR Triage to enhance lung abnormality detection in Samsung's premium X-ray devices SEOUL, South Korea, Jan. 16, 2024 /PRNewswire/ -- Lunit (KRX:328130.KQ), a leading provider of AI-powered solutions for cancer diagnostics and t...
Complete Genomics and Gencove Announce agreement at Plant and Animal Genome Conference (PAG 31) to offer a bundled solution for low-pass whole genome sequencing
SAN JOSE, Calif., Jan. 16, 2024 /PRNewswire/ -- Complete Genomics
Chipscreen NewWay: First Patient Dosed in Phase 1 Trial of the Bispecific Antibody NWY001, a Next-generation Tumor Immunotherapy in Patients with Advanced Solid Tumors
CHENGDU, China, Jan. 16, 2024 /PRNewswire/ -- Chengdu Chipscreen NewWay Biosciences Co., Ltd. (NewWay) announced that onJanuary 5, 2024, the dosing of the first patient for a phase I clinical trial of a PD-1/CD40 bispecific antibody (bsAb), NWY001, at Sun Yat-Sen University Cancer Center inChina,...
PranaQ to Unveil Revolutionary Sleep Tech at CES 2024: Introducing TipTraQ, the AI Sleep Tracking and Improvement Solution
NEW YORK, Jan. 15, 2024 /PRNewswire/ -- PranaQ, the innovative sleep tech startup, has made waves at CES 2024 with the highly anticipated launch of its flagship product, TipTraQ. This cutting-edge device goes beyond traditional sleep monitoring, offering a comprehensive solution for diagnosing an...
CARsgen's CT011 achieves IND clearance from the NMPA for the GPC3-positive stage Ⅲa hepatocellular carcinoma at high risk of recurrence after surgical resection
SHANGHAI, Jan. 15, 2024 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, announces that CT011, an autologous CAR T-cell product candidate against Glypic...
Positive Outcome from a Phase Ib Study in Australia Treating Patients with Androgenic Alopecia
SHANGHAI, Jan. 14, 2024 /PRNewswire/ -- Hope Medicine Inc. ('HopeMed'), a clinical-stage innovative biopharmaceutical company, announced recently that the company has completed a Phase Ib study, "An Open-Label Study, to Evaluate Safety, Tolerability, and Efficacy in Male and Female with Androgene...
San Francisco Witnesses the Voice of GCT Titans GenScript Biotech Global Forum Outlines the Future of the Industry
SAN FRANCISCO, Jan. 12, 2024 /PRNewswire/ -- Every January, the global medical health and financial investment sectors turn their focus toSan Francisco. The J.P. Morgan Healthcare Conference has been successfully held here for 42 editions, becoming a hall where industry thought converges and spar...
Qilian International Holding Group Receives 180-day Extension from Nasdaq to Meet Minimum Bid Price Rule
JIUQUAN, China, Jan. 12, 2024 /PRNewswire/ -- Qilian International Holding
Group Limited (NASDAQ: QLI
ClinChoice Acquires CSI, Expanding its Global Footprint into Southeast Asia
SHANGHAI, PHILADELPHIA and SINGAPORE, Jan. 11, 2024 /PRNewswire/ -- ClinChoice, a leading global Contract Research Organization (CRO), today announced expanding its global footprint with the acquisition of CSI Medical Research (CSI). CSI is a regional clinical CRO with professional staff and part...
United Imaging Intelligence Highlights Dedications to Advancing Intelligent Healthcare at RSNA Conference 2023
BOSTON, Jan. 11, 2024 /PRNewswire/ -- The Radiological Society of North America (RSNA) has concluded its annual meeting inChicago, IL, serving as a platform for revolutionary advancements in the field of radiology. United Imaging Intelligence (UII), an innovative subsidiary of United Imaging Grou...
GenFleet Therapeutics Announces GFH009 Granted with FDA Fast Track, Orphan Drug Designations for Treating R/R Peripheral T-cell Lymphomas and Acute Myeloid Leukemia
SHANGHAI, Jan. 11, 2024 /PRNewswire/ -- GenFleet Therapeutics, a clinical-stage biotechnology company developing cutting-edge therapies in oncology and immunology, announced that U.S. Food and Drug Administration (FDA) has recently granted another two designations for SLS009 (GFH009). GFH009 (hig...
CEO of Kelun-Biotech Dr. Micheal Ge delivered a presentation on The 42nd JPM Annual Healthcare Conference
SAN FRANCISCO, Jan. 11, 2024 /PRNewswire/ -- The 42nd J.P. Morgan Annual Healthcare Conference("JPMHC")2024 is taking place onJanuary 8-11 at San Fracisco,USA, members of Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (6990.HK, Kelun-Biotech) senior management team participated in the conferen...
SyntheticGestalt and Enamine to Collaborate on the Creation of AI-Based Model to Facilitate Drug Discovery
SyntheticGestalt will develop a pre-trained AI model to discover synthesizable drug-like hit candidates. This model will utilize 38 billion compounds from Enamine REAL database as a dataset for pre-training. TOKYO and KYIV, Ukraine, Jan. 11, 2024 /PRNewswire/ -- SyntheticGestalt, a research and ...
Ascentage Pharma Presented at 42nd Annual J.P. Morgan Healthcare Conference
SUZHOU, China and ROCKVILLE, Md., Jan. 10, 2024 /PRNewswire/ -- Ascentage Pharma (6855.HK), a commercial stage global biopharmaceutical company engaged in developing novel therapies for cancer, chronic hepatitis B (CHB), and age-related diseases, announced today that Dr.Dajun Yang, the company's ...
Robooter Showcases Innovative Power Wheelchair X40 at CES 2024
LAS VEGAS, Jan. 10, 2024 /PRNewswire/ -- Robooter, a leading autonomous mobility device manufacturer renowned for its robotic technology supporting the elderly and disabled, is proud to present the innovative power wheelchair X40 and offer a new space and mobility solution through its partners at...
New Chinese medicine drug developed by HKBU for myofibrillar myopathy granted orphan drug designation by FDA
HONG KONG, Jan. 10, 2024 /PRNewswire/ -- The Centre for Chinese Herbal Medicine
Drug Development
LOTTE BIOLOGICS Announces Songdo Bio Plant Development Plan at JPM Healthcare Conference 2024
* Richard Lee, CEO of LOTTE BIOLOGICS, delivers a keynote speech at the JPM Healthcare Conference for the second consecutive year * Unveils company's bio plant development plan in Songdo to meet clients' needs SEOUL, South Korea, Jan. 9, 2024 /PRNewswire/ -- LOTTE BIOLOGICS (CEO Richard Lee) ...
Week's Top Stories
Most Reposted
Wonder Raises USD 12 Million Venture Debt from HSBC Innovation Banking to Drive Growth and Expansion
[Picked up by 322 media titles]
2026-02-02 10:00Agoda Launches Agoda Impact Lab at ASEAN Tourism Forum
[Picked up by 322 media titles]
2026-01-29 15:06AI adoption is widespread, but developer confidence is still catching up, Agoda report finds
[Picked up by 312 media titles]
2026-02-03 11:00Mastercard Launches Portfolio of Fleet Solutions in Asia Pacific
[Picked up by 308 media titles]
2026-02-04 09:00Colebrook Bosson Saunders Officially Launches Lana, A Circular Ergonomic Laptop Stand for the Hybrid Generation
[Picked up by 303 media titles]
2026-02-03 12:00