Medical/Pharmaceuticals
PRISM BioLab, enters multi-project drug discovery collaboration with Roche and Genentech
TOKYO, Jan. 4, 2022 /PRNewswire/ -- PRISM BioLab, a Japan based biotechnology company with a proprietary peptide mimetic small molecule drug discovery platform, today announced it has entered into a multi-target research collaboration and licensing agreement with Roche and Genentech, a member of ...
BIORCHESTRA, SK Biopharmaceuticals collaborate to develop miRNA-targeted therapeutics
SEOUL, South Korea, Jan. 4, 2022 /PRNewswire/ -- BIORCHESTRA and SK
Biopharmaceuticals announcedtoday that they have forged a partnership to
research and develop new therapeutic compounds targeting microRNAs [1] (miRNA)
for a potential treatment of epilepsy.
Harbour BioMed Announces Dosing of First Patient of Two Phase I Trials for Next-Generation Anti-CTLA-4 Antibody HBM4003
CAMBRIDGE, Mass. and ROTTERDAM, Netherlands and SUZHOU, China, Jan. 4, 2022 /PRNewswire/ -- Harbour BioMed ("HBM", HKEX: 02142) announced that, its next generation anti-CTLA-4 fully human heavy-chain only antibody (HCAb), HBM4003, has completed the first dosing of first patient in two phase I tri...
AKESO'S CADONILIMAB (PD-1/CTLA-4 BI-SPECIFIC ANTIBODY) COMBINED WITH CONCURRENT CHEMORADIOTHERAPY OBTAINED APPROVAL TO INITIATE A PHASE III CLINICAL TRIAL FOR THE TREATMENT OF LOCALLY ADVANCED CERVICAL CANCER
HONG KONG, Jan. 3, 2022 /PRNewswire/ -- Today, Akeso (09926.HK) announces that Cadonilimab (PD-1/ CTLA-4 bi-specific antibody), the first-inclass novel immuno-oncology drug independently developed by the Company, combined with concurrent chemoradiotherapy obtained approval from the Center for Dru...
Asieris Announces the World's First Patient Dose Administered in Combination of Asieris'APL-1202 and BeiGene's Tislelizumab as Neoadjuvant Therapy for MIBC Patients
SHANGHAI, Jan. 3, 2022 /PRNewswire/ -- Asieris Pharmaceuticals (Asieris), a global innovative pharma company specializing in new drugs for the treatment of genitourinary tumors, today announced that the world's first patient dose has been administered in U.S. for its oral APL-1202 in combination ...
Biosion, Inc. Appoints Anthony Yeh, Ph.D., as Chief Strategy Officer and Head of China BD
NEWARK, Del. and NANJING, China, Jan. 3, 2022 /PRNewswire/ -- Biosion, Inc. ("Biosion"), a global clinical stage biotechnology company, today announced the appointment of Dr.Anthony Yeh to the position of Chief Strategy Officer and Head ofChina business development (BD), where he will lead corpor...
Pulnovo Medical Announced Results From PADN-CFDA Pivotal Trial For The Treatment Of Pulmonary Arterial Hypertension (PAH) Meet The Efficacy Primary Endpoint
SHANGHAI, Jan. 1, 2022 /PRNewswire/ -- Pulnovo Medical Limited, a globally recognized device pioneer in treatment for cardiopulmonary disease, today announced the positive results from the Pulmonary Artery Denervation (PADN)-CFDA pivotal study, being the first global completed pulmonary hyperten...
I-Mab Announces Upcoming Participation at January Conferences
SHANGHAI and GAITHERSBURG, MD., Dec. 31, 2021 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today announced its participation in the following conferences inJanuar...
China Medical System (867.HK)'s Latest MSCI ESG Rating Unchanged at AA, Leading the Industry Globally
HONG KONG, Dec. 30, 2021 /PRNewswire/ -- Recently, Morgan Stanley Capital International (MSCI), the world's largest index provider, has updated the Environmental, Social and Governance (ESG) rating of China Medical System Holdings Limited ("CMS" or the "Company").CMS's latest rating remains uncha...
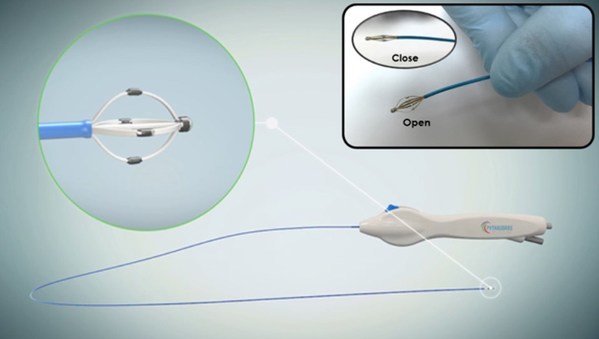
Peijia Medical's New Milestone Asia's first successful clinical case using HighLife transseptal mitral valve replacement technology
SUZHOU, China, Dec. 30, 2021 /PRNewswire/ -- On December 22, 2021, the first clinical case using HighLife transseptal mitral valve replacement ("TSMVR") technology was successfully carried out inAsia. The implantation of Peijia's Highlife TSMVR system was performed by ProfessorMao Chen and his te...
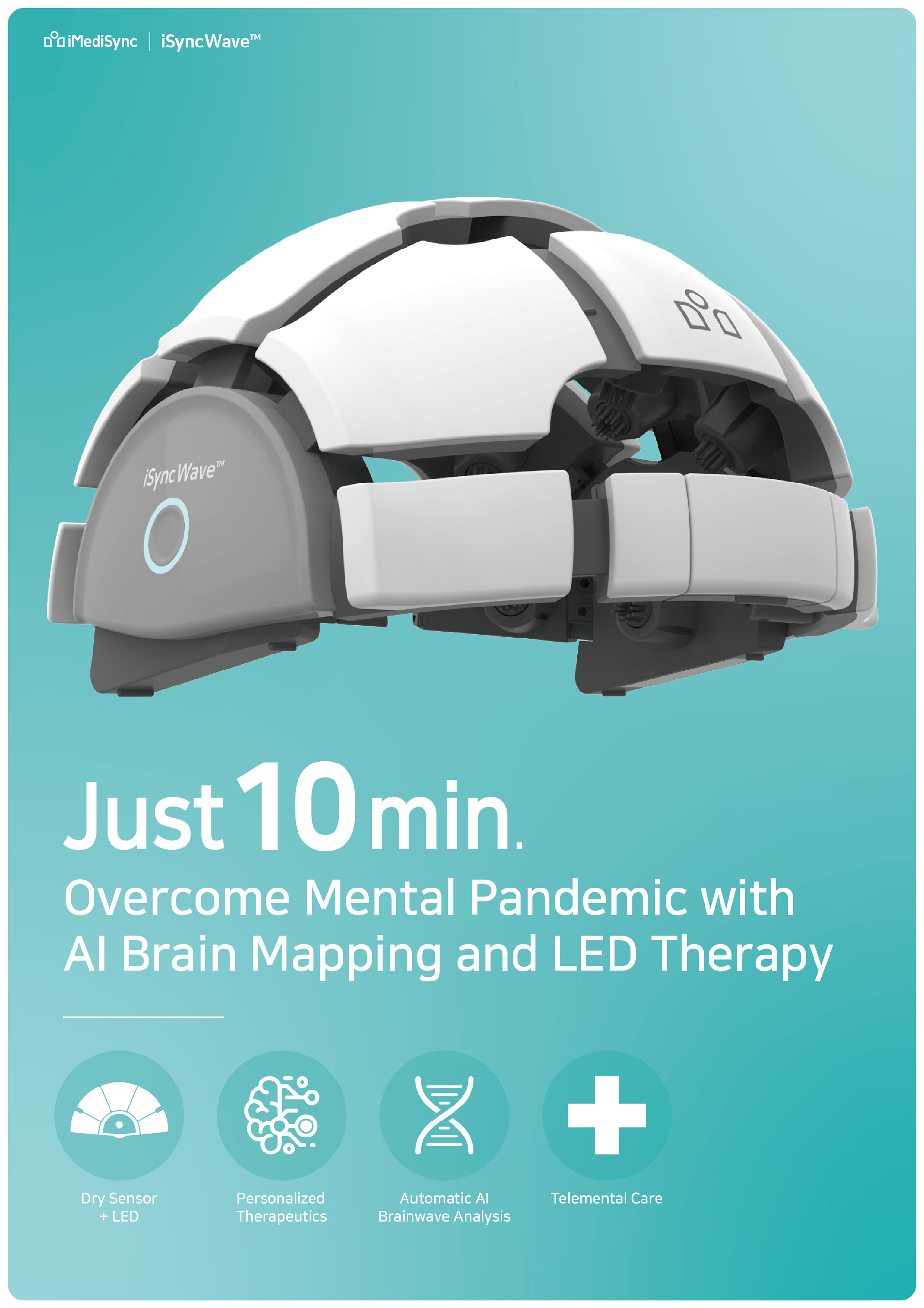
iMEDISYNC UNVEILS WORLD'S FIRST INTEGRATED WIRELESS BRAIN EEG at CES 2022
SEOUL, South Korea, Dec. 29, 2021 /PRNewswire/ -- iMediSync, a leading AI-driven early detection and therapeutic platform for optimal brain health from Seoul, South Korea announced today that the company will showcase its comprehensive EEG solution (hardware + software + remote telehealth solution...
SyMap Medical Ltd. Announces its Strategic Collaboration with Pythagoras Medical
SUZHOU, China, Dec. 29, 2021 /PRNewswire/ -- SyMap Medical Ltd (SyMap) announced its strategic collaboration with Pythagoras Medical. With this win-win collaboration, SyMap has full rights to utilize all assets of Pythagoras, including their Renal Nerve Mapping System (ConfidenHT™, CE Mark Appro...
EOFlow and Zihipp Announce Joint Venture for Development of Innovative Solutions for Treatment of Obesity & NASH
* Establishment of Joint Venture Company named SanPlena in the US * Drug-device combination of EOFlow's smart wearable drug delivery platform and novel peptide derivatives developed by a team led by Prof. SirStephen Bloom , world-renowned researcher in the field of obesity and metabolism at Imp...
Akeso's IL-17A MONOCLONAL ANTIBODY (GUMOKIMAB) COMPLETION OF PATIENT ENROLLMENT IN PHASE II CLINICAL TRIAL FOR THE TREATMENT OF ANKYLOSING SPONDYLITIS
HONG KONG, Dec. 27, 2021 /PRNewswire/ -- Today, Akeso, Inc. (09926.HK) announces that Gumokimab (IL-17A monoclonal antibody, AK111), an innovative drug independently developed by the Company for the treatment of active ankylosing spondylitis has been completed. Such clinical trial aims to evalua...
Regor Therapeutics Announces U.S. FDA Authorization to Conduct Regor's First-in-Human Clinical Trial with the Next Generation Targeted Inhibitor RGT-419B for Oncology
SHANGHAI, Dec. 27, 2021 /PRNewswire/ -- On December 23, Regor Therapeutics, a clinical-stage biotech company,announced authorization from the US Food and Drug Administration (FDA) to proceed with Regor's Phase 1 clinical development plans for RGT-419B. RGT-419B is a new generation CDK2/4/6, smal...
I-Mab Announces IND Approval from China NMPA for Phase 2 Clinical Trial of Enoblituzumab in Combination with Pembrolizumab in Solid Tumors
SHANGHAI and GAITHERSBURG, Md., Dec. 27, 2021 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, announced that the Center for Drug Evaluation (CDE) ofChina's National ...
Kintor Pharma Provides Update on One of its Three Multi-Regional Phase 3 Trials of Proxalutamide for COVID-19
SUZHOU, China, Dec. 27, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited ("Kintor Pharma," HKEX: 9939), a clinical-stage biotechnology company developing innovative small molecule and biological therapeutics, today provided an update on its multi-regional study of proxalutamide for the treatme...
Laekna Presents Vision and Strategy at NewYorkBIO/NYSE Emerging Company Showcase
SHANGHAI and WARREN, N.J., Dec. 24, 2021 /PRNewswire/ -- Laekna was invited by NewYorkBIO and the New York Stock Exchange (NYSE) to present, as an emerging biotech company at the "Emerging Company Showcase" that was held earlier this month inNew York. Dr. Guy Rosenthal, Vice President, Head of C...
Curocell, Breaks Ground on 'CAR-T Manufacturing GMP Facility'
DAEJEON, South Korea, Dec. 23, 2021 Curocell, currently on PhaseⅠCAR-T Therapy
clinical trial withCRC01 (anbalcabtagene autoleucel) has recently broken ground
for the new CAR-T Center in Dungok Residential & Industrial Area in Daejeon
International Science & Business Belt.
Akeso's PCSK9 MONOCLONAL ANTIBODY (EBRONUCIMAB) EARLY COMPLETION OF PATIENT ENROLLMENT IN A PHASE III CLINICAL TRIAL FOR PRIMARY HYPERCHOLESTEROLEMIA AND MIXED HYPERLIPIDEMIA
HONG KONG, Dec. 22, 2021 /PRNewswire/ -- Today, Akeso, Inc. (09926.HK) announces that Ebronucimab (PCSK9 monoclonal antibody, research and development code: AK102), jointly developed by the Company and Dawnrays Biotechnology Capital (Asia) Ltd., completed patient enrollment early in a pivotal re...
Week's Top Stories
Most Reposted
Agoda Launches Free Global eSIMs for VIP Diamond Members
[Picked up by 322 media titles]
2026-02-10 14:00Ascentium Acquires Clara, Expanding into the Abu Dhabi Global Market (ADGM) and Strengthening its Middle East Presence
[Picked up by 310 media titles]
2026-02-12 14:00Rockwell Automation Strengthens Industrial Cybersecurity with New Security Operations Center in Singapore
[Picked up by 298 media titles]
2026-02-09 10:00Blackpanda Japan Announces Strategic Partnership with SoftBank to Strengthen Cyber Incident Response in Japan
[Picked up by 298 media titles]
2026-02-10 13:31Carro unveils quirky generative AI ad campaign highlighting its 'Surprisingly Short' AI-enabled car-selling process
[Picked up by 296 media titles]
2026-02-11 11:00