Medical/Pharmaceuticals
China Pharmaceutical Holdings Co., Ltd. Added Mask Production Lines
HAIKOU CITY, China, April 15, 2020 /PRNewswire/ -- China Pharmaceutical Holdings Limited (NYSE American: CPHI) ("China Pharma" or "Company"), an NYSE American-listed corporation with a fully-integrated specialty pharmaceuticals subsidiary based inChina, today announced that its subsidiary, Hainan...
Updates - China Pharma Holdings, Inc. Reports Fiscal Year 2019 Financial Results
HAIKOU CITY, China, April 14, 2020 /PRNewswire/ -- China Pharma Holdings, Inc. (NYSE American: CPHI) ("China Pharma," the "Company" or "We"), an NYSE American-listed corporation with a fully-integrated specialty pharmaceuticals subsidiary based inChina, today announced that it is re-issuing its ...
Nucleus Network's 10 Step Guide to conducting Clinical Trials during a time of uncertainty
MELBOURNE, Australia, April 14, 2020 /PRNewswire/ -- A new white paper,
Undertaking Phase I Clinical Trials inAustralia During a Time of Uncertainty
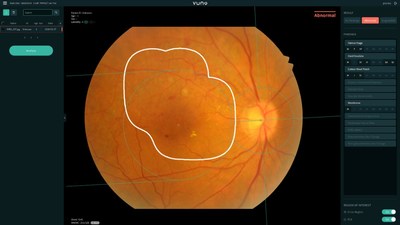
VUNO Med(R)-Fundus AI(TM) receives MFDS Regulatory Approval as Class III Medical Device
SEOUL, South Korea, April 13, 2020 /PRNewswire/ -- VUNO, a member company of the Born2Global Centre, announced that their AI based screening solution for the fundus, VUNO Med®-Fundus AI™ is approved as a Class III medical device by the Ministry of Food and Drug Safety. VUNO Med®-Fundus AI™ is the f...
Gracell to Present the First-in-human, Universal TruUCAR™ GC027 Therapy for Relapsed or Refractory T-cell Acute Lymphoblastic Leukemia at the AACR Annual Meeting
SUZHOU, China and SHANGHAI, April 13, 2020 /PRNewswire/ -- Gracell Biotechnologies Co., Ltd. ("Gracell"), a clinical-stage immune cell and gene therapy company, is pleased to announce that their first-in-human phase I data of UniversalTruUCAR™ GC027 in relapsed or refractory (R/R) T-cell acute l...
EmeTerm, by WAT Medical, an easy solution for Cyber Sickness
VANCOUVER, April 13, 2020 /PRNewswire/ -- WAT Medical Enterprise Ltd. conducted an investigation on solutions to cyber sickness (a form of nausea-induced vomiting). WAT Medical team has been studying has achieved positive results with the use of EmeTerm. Based on a large number of experimental te...
Samsung Biologics and Vir Biotechnology Enter into Agreement for Large Scale Manufacture of SARS-COV-2 Antibodies for Potential COVID-19 Treatment
SONGDO, South Korea and SAN FRANCISCO, April 9, 2020 /PRNewswire/ -- Samsung Biologics (207940.KS) and Vir Biotechnology, Inc. (Nasdaq: VIR) today announced a manufacturing agreement under which Samsung Biologics will perform large scale manufacturing services for Vir's SARS-CoV-2 monoclonal anti...
Bridge Biotherapeutics Discloses Interim Results from the First Cohort of Phase 2a Study of BBT-401, an Experimental Drug for Ulcerative Colitis
* The Interim data showed that 3 out of 9 evaluable patients on BBT-401 responded with more than 30% reduction in Total Mayo Score or 25% reduction in Partial Mayo Score * Safety and tolerability profiles remain consistent with results from Phase I trial in healthy volunteers SEONGNAM, South ...
United Imaging Announces Rapid CT Deployment to Help Expand Capacity at Maimonides Medical Center
HOUSTON, April 8, 2020 /PRNewswire/ -- United Imaging, a global leader in advanced medical imaging and radiotherapy equipment, announced that it has installed a transportable CT scanner at the request of Maimonides Medical Center, the largest hospital inBrooklyn, New York, on the front lines of t...
Samsung Biologics named Champion in 2020 CMO Leadership Awards
SONGDO, South Korea, April 8, 2020 /PRNewswire/ -- Samsung Biologics (207940.KS) announced that it has been awarded the 2020 CMO Leadership Awards for its excellence in the Capabilities, Compatibility, Quality, Reliability, and Service categories across Big Pharma and Overall (combined Big and Sm...
China-based Asclepius Metidec helps solve ventilator shortage with its innovative device
SHANGHAI, April 8, 2020 /PRNewswire/ -- An innovative technology-powered medical device developed by Chinese medical equipment provider Asclepius Metidec has been successfully used in the treatment of patients with confirmed COVID-19. The hydrogen oxygen nebulizer, as a result of nine years of r...
From China to the World, Fosun's Fight against COVID-19 Continues
SHANGHAI, April 7, 2020 /PRNewswire/ -- At 7:00 pm Beijing time on April 6,
Fosun hosted a global virtual conference on fighting against COVID-19 on the
39th floor of Fosun's headquarters - the Bund Finance Center inShanghai.
GenScript ProBio and Eutilex Enter into Exclusive Strategic Collaboration on Plasmid and Virus Process Development and Manufacturing for CAR-T Programs
NANJING, China and SEOUL, South Korea, April 7, 2020 /PRNewswire/ -- GenScript Biotech Corporation's CDMO business GenScript ProBio (hereinafter referred to as GenScript ProBio) and Eutilex Co., Ltd. (hereinafter referred to as Eutilex) committed to developing anti-cancer immunotherapy technology...
Yuyu Pharma appoints Robert Wonsang Yu as incoming President and CEO
SEOUL, South Korea, April 5, 2020 /PRNewswire/ -- Yuyu Pharma (KRX: 000220), a
global pharmaceutical company based in Korea, is pleased to announce the
appointment of Mr.Robert Wonsang Yu as President and Chief Executive Officer
effectiveApril 6, 2020.
Samsung Biologics enters into a development and manufacturing partnership with PharmAbcine for oncology and neovascular treatment
SONGDO, South Korea, April 5, 2020 /PRNewswire/ -- Samsung Biologics (207940.KS) entered into a strategic partnership with PharmAbcine for the development and manufacturing of PMC-402 pipeline, a next generation therapeutic antibody candidate to treat oncology and neovascular disorders. Under th...
United Imaging Releases Information on Additional Transportable CTs for the U.S.
HOUSTON, April 5, 2020 /PRNewswire/ -- United Imaging, a global leader in advanced medical imaging and radiotherapy equipment, followed a recent March announcement about transportable CTs with more details on a second collaboration. Continuing its focus on deploying scanners in portable structur...
Gracell Announces China NMPA Acceptance of Investigational New Drug Application for GC007g Cell Therapy for CD19 Positive Relapsed or Refractory B-cell Acute Lymphoblastic Leukemia
SHANGHAI and SUZHOU, China, April 3, 2020 /PRNewswire/ -- Gracell Biotechnologies Co., Ltd. ("Gracell"), a clinical-stage immune cell & gene therapy company, is pleased to announce that China National Medical Products Administration (NMPA) has accepted Gracell's Investigational New Drug (IND) ...
VUNO offers a suite of AI solutions in response to the COVID-19 outbreak: VUNO Med(R)- LungQuant(TM) and VUNO Med(R)-Chest X-ray(TM): COVID-19
SEOUL, South Korea, April 3, 2020 /PRNewswire/ -- In the face of the COVID-19 pandemic raging around the globe,VUNO, a member company of the Born2Global Centre, has decided to move quickly by joining the global coalition to help address some critical issues facing the international healthcare com...
SonoScape supports frontline battle against COVID-19 in Spain
MADRID, April 3, 2020 /PRNewswire/ -- SonoScape, as one of the leading providers of medical ultrasound and endoscopy solutions, has donated and delivered portable ultrasound systems to health care facilities where needed in Spain, pulling together to fight against COVID-19. COVID-19 has a foothol...
Jolly Good and National Center for Cognitive Behavior Therapy and Research Enter Clinical Trial Collaboration for VR Therapeutics
TOKYO, April 2, 2020 /PRNewswire/ -- Jolly Good Inc. (hereinafter "Jolly Good") and the National Center for Cognitive Behavior Therapy and Research (hereinafter "NCCBTR") announced that they have entered into a clinical trial collaboration agreement directed toward the development of digital heal...
Week's Top Stories
Most Reposted
UTP LAUNCHES MALAYSIA'S FIRST BACHELOR IN INTEGRATED ENGINEERING PROGRAMME
[Picked up by 314 media titles]
2024-12-27 13:17COCONEXT 2024 - FIRST EVER INTERNATIONAL COCONUT CONFERENCE HOLD IN VIETNAM
[Picked up by 291 media titles]
2024-12-31 14:18Dahua Technology Obtains ISO 37301 Compliance Management System Certification
[Picked up by 275 media titles]
2024-12-30 15:33ebm-papst A&NZ Achieves Complete Operational Carbon Neutrality in appropriate Scope 1,2 and 3 criterion in 2023
[Picked up by 250 media titles]
2024-12-25 15:45CKGSB Professor Mei Jianping Launches Global Indices Tracking Impressionist, Contemporary, and Chinese Art Markets
[Picked up by 247 media titles]
2024-12-31 22:30