Medical/Pharmaceuticals
Chipscreen's New Drug Candidate CS32582 Capsules Approved for Clinical Treatment of Psoriasis
SHENZHEN, China, Nov. 2, 2023 /PRNewswire/ -- On Oct. 31, 2023, Shenzhen Chipscreen Biosciences Co., Ltd. (hereinafter referred to as "Chipscreen" and with stock code 688321.SH) received the "Drug Clinical Trial Approval Notice" issued by the National Medical Products Administration ("NMPA") thro...
Precision, Effectiveness, and Intelligence: Explore the Latest Trend of Healthcare and Medical Equipment Industry at 134th Canton Fair
GUANGZHOU, China, Nov. 1, 2023 /PRNewswire/ -- As the public is paying increasing attention to health management, the 5-day onsite exhibition of Phase 3 of the 134th Canton Fair held from October 31st to November 4th will present nearly 600 quality exhibitors in medical and healthcare industries ...
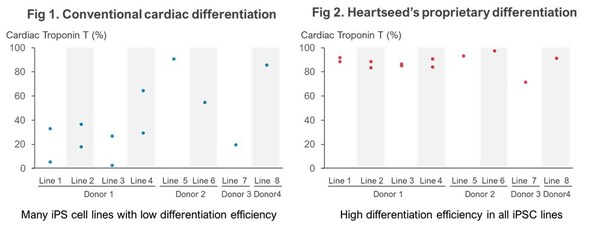
Using iPS Cells from I Peace, Heartseed Succeeds in Stable Production of High Purity Cardiomyocytes, A Major Step Forward in Advancing Autologous Cardiac Regenerative Medicine
PALO ALTO, Calif., Nov. 1, 2023 /PRNewswire/ -- Leading GMP cell CDMO I Peace,
Inc. (https://www.ipeace.com
AI-Powered Tumor Microenvironment Analysis Predicts Treatment Outcomes in NSCLC Patients with EGFR Mutation: Groundbreaking Studies to be Presented by Lunit at SITC 2023
* Six posters featuring the Lunit SCOPE suite and new insights into the tumor microenvironment are to be showcased at the SITC 2023 Annual Meeting SEOUL, South Korea, Nov. 1, 2023 /PRNewswire/ -- Lunit (KRX:328130.KQ), a leading provider of AI-powered solutions for cancer diagnostics and therap...
Jolly Good Inc. Establishes North American Subsidiary: Joint Development of Medical VR for "Apple Vision Pro" with U.S. Experts
~ First Phase: Joint Development of Chronic Pain VR with U.S. Chronic Pain Experts TOKYO, Nov. 1, 2023 /PRNewswire/ -- Jolly Good Inc. (Chuo-ku, Tokyo, CEO: Kensuke Joji, hereinafter referred to as Jolly Good), which develops and provides medical VR, announces that it has established a North Ame...
I-Mab Announces Poster Presentations of 4-1BB Bispecific Antibody Portfolio at SITC 2023
ROCKVILLE, MD, U.S. and SHANGHAI, China, Nov. 1, 2023 /PRNewswire/ -- I-Mab (Nasdaq: IMAB) (the "Company"), a global biotechnology company focused on bringing highly differentiated medicines to patients around the world through the discovery, development, and commercialization of novel immunother...
Curocell Completes Korea's First Phase 2 Clinical Trial for Next-Generation CAR-T
* Anbal-cel targeting DLBCL completes phase 2 trial * Anticipation growing over Korea's first CAR-T therapy ahead of regulatory review * Commercial manufacturing scheduled in 2025 at Korea's only CAR-T GMP facility DAEJEON, South Korea, Nov. 1, 2023 /PRNewswire/ -- Curocell, South Korea ...
Complete Genomics demonstrates technical and commercial momentum in the sequencing market through new customers, partnerships and collaborations less than one year after launching in the U.S.
WASHINGTON, Oct. 31, 2023 /PRNewswire/ -- Complete Genomics, a pioneering genomic sequencing company, announced today at the American Society of Human Genetics (ASHG) Annual Meeting,Nov. 1-5, in Washington details on the commercial and technical momentum it has demonstrated in the last 10 months ...
Lunit Joins as an Associate Partner with the World Economic Forum
- Lunit joins as an Associate Partner of the World Economic Forum, propelling global expansion and collaboration in the realm of AI-driven cancer care SEOUL, South Korea, Oct. 31, 2023 /PRNewswire/ -- Lunit (KRX:328130.KQ), a leading provider of AI-powered solutions for cancer diagnostics and th...
Meihua International Medical Technologies Co., Ltd. Establishes A Subsidiary Dedicated to Introducing International Patented Pharmaceuticals and Medical Device Technologies to China and Hainan Free Trade Port Boao Hope City
YANGZHOU, China, Oct. 31, 2023 /PRNewswire/ -- Meihua International Medical Technologies Co., Ltd. ("MHUA" or the "Company") (NASDAQ: MHUA), a reputable manufacturer and provider of Class I, II and III disposable medical devices with operating subsidiaries inChina, announced today the establishme...
CHIMERIC THERAPEUTICS ANNOUNCES FDA CLEARANCE OF IND APPLICATION FOR CHM 2101, A NOVEL CDH17 CAR T CELL THERAPY FOR ADVANCED GASTROINTESTINAL CANCERS
* FDA IND Clearance for CHM 2101, a novel 3rd generation CDH17 CAR T cell therapy * Anticipated to be the first CDH17 CAR T cell therapy to enter the clinic * Phase 1A clinical trial to initiate patient enrolment in 2024 * Phase 1A clinical trial will enroll patients with advanced Colorecta...
iNtRON, Executes Evaluation License and Option Agreement for SAL200
* iNtRON Grants Basilea exclusive right for preclinical evaluation with an undisclosed payment * Basilea has the option to enter into exclusive License Agreement following the evaluation BOSTON and SEOUL, South Korea, Oct. 30, 2023 /PRNewswire/ -- iNtRON Biotechnology ("iNtRON",www.intodeworl...
Bridge Biotherapeutics Announces Initiation of Phase 1/2 Clinical Trial of BBT-207 in EGFR-Mutant NSCLC
SEONGNAM, South Korea and NEWTON, Mass., Oct. 30, 2023 /PRNewswire/ -- Bridge Biotherapeutics (KQ288330), a South Korean clinical-stage biotech company developing novel drugs for cancer, fibrosis, and inflammation, announced that the company has initiated the Phase 1/2 clinical trial evaluating t...
Pulsecare Medical's nsPFA clinical trial receives satisfactory short-term follow-up results
SHENZHEN, China, Oct. 30, 2023 /PRNewswire/ -- Pulsecare Medical, an innovative minimally invasive and non-invasive therapeutic technology company, announced today that its nanosecond pulsed field ablation (nsPFA) system for cardiac electrophysiology, the world's first third-generation pulsed fie...
AIRS Medical Accelerates Global Expansion, Showcasing clinical achievements of MRI Enhancement Solution at RSNA 2023
* Recently achieved MDSAP certification and ISO/IEC 27001, 27017, 27018 certifications, paving the way for accelerated expansion into global markets. * Certified Japan's Pharmaceuticals and Medical Devices Agency (PMDA), signaling the beginning of its full-scale expansion into the Japanese mar...
WuXi AppTec Continued Solid Growth in the First Three Quarters of 2023 on Top of an Exceptionally Strong Year in 2022, with Profit Growth Continuously Exceeding Revenue Growth
* Revenue of RMB10,670 Million in the Third Quarter, Single Quarter Revenue Back to overRMB10 Billion; Revenue of RMB29,541 Million in the First Three Quarters, Up 4.0% Year-over-Year; Excluding COVID-19 Commercial Projects, Up 23.4% * Net Profit Attributable to Owners of the Company for the ...
Journal of Thoracic Oncology Published Promising Results of Ivonescimab (PD-1/VEGF Bispecific) as First- or Second-line Therapy for Advanced or Metastatic Immunotherapy Naïve Non-Small-Cell Lung Cancer
HONG KONG, Oct. 30, 2023 /PRNewswire/ -- Akeso (9926.HK) announced that the results of a phase Ib clinical trial for PD-1/VEGF bispecific antibody ( ivonecimab AK112/SMT112 ) as first- or second-line therapy for advanced or metastatic immunotherapy naïve non-small-cell lung cancer (NSCLC) were p...
Foresee Pharmaceuticals to Present at the American Heart Association Annual Meeting Focusing on its ALDH2 activator FP-045 in Pulmonary Hypertension Associated with Interstitial Lung Disease
TAIPEI, Oct. 30, 2023 /PRNewswire/ -- Foresee Pharmaceuticals (TPEx: 6576), ("Foresee") announced today that the company will be presenting at the American Heart Association (AHA) 2023 Annual Meeting taking place inPhiladelphia, PA, fromNovember 11-13, 2023. The presentation will focus on Foresee...
Lunit Enters into Research Collaboration to Explore the Use of AI to Improve the Effectiveness of Immunotherapy
- Lunit to support MD Anderson researchers studying immune phenotype biomarkers for pembrolizumab treatment response SEOUL, South Korea, Oct. 27, 2023 /PRNewswire/ -- Lunit (KRX:328130.KQ), a leading provider of AI-powered solutions for cancer diagnostics and therapeutics, today announced ...
Peijia Medical Presents Early Clinical Findings of GeminiOne® TEER Device at TCT 2023
SUZHOU, China, Oct. 27, 2023 /PRNewswire/ -- Peijia Medical Limited (Peijia, (9996.HK)), a leading Chinese medical device player in transcatheter structural heart device and neurovascular device, presented its GeminiOne® TEER technology, along with the early clinical experiences of the device at ...
Week's Top Stories
Most Reposted
Wonder Raises USD 12 Million Venture Debt from HSBC Innovation Banking to Drive Growth and Expansion
[Picked up by 322 media titles]
2026-02-02 10:00AI adoption is widespread, but developer confidence is still catching up, Agoda report finds
[Picked up by 313 media titles]
2026-02-03 11:00Mastercard Launches Portfolio of Fleet Solutions in Asia Pacific
[Picked up by 310 media titles]
2026-02-04 09:00Colebrook Bosson Saunders Officially Launches Lana, A Circular Ergonomic Laptop Stand for the Hybrid Generation
[Picked up by 304 media titles]
2026-02-03 12:00Singapore Airshow 2026 Milestone Edition: 20 Years of Shaping the Aerospace Landscape as Asia-Pacific Drives Global Growth
[Picked up by 287 media titles]
2026-02-01 19:35