Medical/Pharmaceuticals
Henlius Deepens Collaboration with Intas to bring Henlius' Novel anti-PD-1 mAb Serplulimab to Europe and India
* The footprint of serplulimab now includes the United States, Europe, Southeast Asia, MENA, and India * Intas to develop and commercialise serplulimab in Europe and India; Henlius to receive €42 million upfront payment, double-digit royalties and up to €143 million in regulatory and commerc...
Chime Biologics and Hope Medicine Enter Manufacturing Agreement to Speed up the Launch of First-in-class Antibody Drug HMI-115 Targeting Endometriosis and Androgenic Alopecia
* Chime Biologics to support the late-stage clinical study and to provide global commercial manufacturing services forHope Medicine's first-in-class monoclonal antibody drug, HMI-115. * The first-in-class mAb will benefit endometriosis and androgenetic alopecia patients. SHANGHAI, Oct. 26, 20...
Clarity and PSI kick off SAR-bisPSMA Phase III
SYDNEY, Oct. 26, 2023 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, and PSI CRO AG ("PSI"), a global contract rese...
HanAll Biopharma Reports Third Quarter 2023 Financial Results and Provides a Business Update
* Sales recorded KRW 33 billion in Q3 2023, an 11 percent increase from the same period in 2022, due to the sustained growth of major products. * 'HL161ANS', HanAll's second anti-FcRn antibody, demonstrated best-in-class potential through the initial outcome of Phase 1 study. * 'HL192', a Nu...
JINGHAO MEDICAL's self-fitting OTC hearing aids received FDA 510(k) approval
HONGKONG, SHANGHAI, and BEIJING, Oct. 26, 2023 /PRNewswire/ -- As the first hearing aid manufacturer to be listed in China, JINGHAO MEDICAL has once again taken a historic step. OnSeptember 26, 2023, the self-fitting OTC hearing aid developed and manufactured by JINGHAO MEDICAL officially receive...
Bridge Biotherapeutics Announces Positive Recommendation from the Independent Data Monitoring Committee of the BBT-877 Phase 2a Study in Idiopathic Pulmonary Fibrosis
* The independent data monitoring committee (IDMC) recommended the continuation of the BBT-877 Phase 2a trial based on the initial clinical data from the first 20 participants dosed with theinvestigational product * No safety concerns were raised based on the evaluation of the data presented ...
Positive results from first-in-human completely leadless CRT in the US, published in The Journal of the American College of Cardiology
SUNNYVALE, Calif., Oct. 25, 2023 /PRNewswire/ -- EBR Systems, Inc.
Neuragenex Brings Innovative Fibromyalgia Treatment to Patients Across the US
GULBERT, Ariz., Oct. 25, 2023 /PRNewswire/ -- Neuragenex, the national healthcare provider focused on non-pharmaceutical pain treatment, announced its continued expansion to bring relief to fibromyalgia patients across the country. With clinics now operating in Georgia, Texas, Illinois, Tenness...
Qilu Pharmaceutical announces the latest results from its clinical study on QL1706, in combination with chemotherapy, as a first-line treatment for recurrent or metastatic cervical cancer
JINAN, China, Oct. 25, 2023 /PRNewswire/ -- On October 22, Qilu Pharmaceutical unveiled the latest findings from a multicenter, single-arm Phase II clinical trial at the European Society for Medical Oncology (ESMO) Annual Congress 2023. This trial studied the use of QL1706 (iparomlimab and tuvonr...
First Patient Dosed for MRCT Phase 3 Study on First-Line LS-SCLC of Henlius Anti-PD-1 mAb Serplulimab in Europe
SHANGHAI, Oct. 25, 2023 /PRNewswire/ -- Shanghai Henlius Biotech, Inc. (2696.HK) announced that the first patient inEurope has been dosed in the international multi-centre phase 3 clinical trial (NCT05353257) of the company's self-developed anti-PD-1 mAb HANSIZHUANG (serplulimab) in combination ...
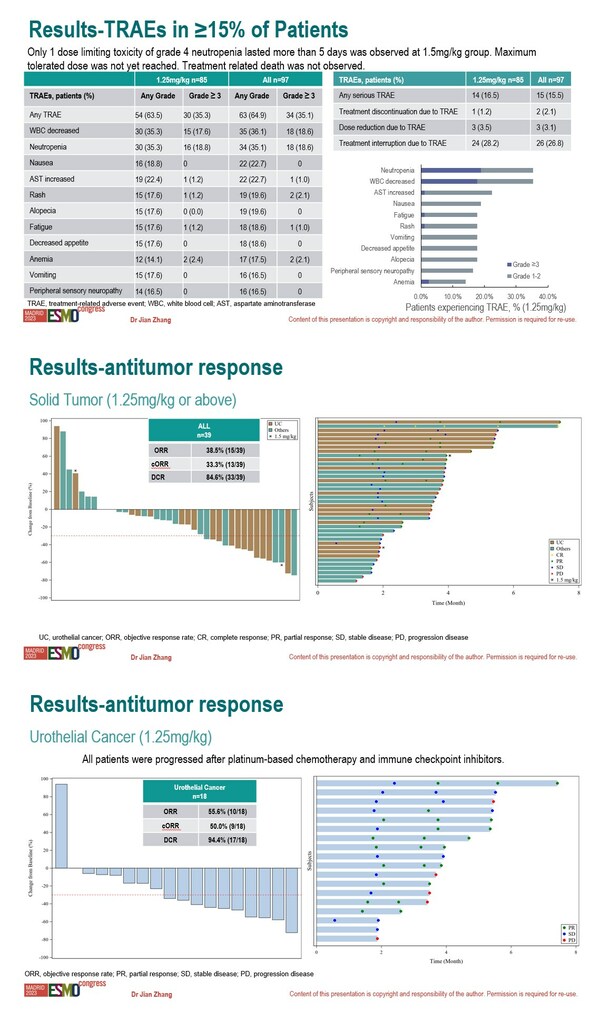
Good Safety Profile and Antitumor Activity: Oral Presentation of Mabwell's 9MW2821 at 2023 ESMO
Shanghai, Oct. 25, 2023 /PRNewswire/ -- ESMO Congress 2023 was held in Madrid, Spain from October 20-24, the preliminary results of the Phase I/II study of 9MW2821 (Nectin-4 targeting ADC) in patients with advanced solid tumors were reported by Dr.Jian Zhang of Fudan University Shanghai Cancer Ce...
GC Cell Joins U.S Cancer Moonshot Project
Brining Expertise in NK Cell Therapy to Advance Efforts in Cancer Prevention, early detection, and treatment development ▶ New focus on NK cell therapy for conquering cancer ▶ GC Cell seizes the opportunity to share its cell gene therapy technology and capabilities globally ▶ Development of sol...
First Immunotherapy Success in TKI-Resistant Lung Cancer Setting Demonstrates Power of AI-driven Immune Phenotyping by Lunit SCOPE IO - newly published in JCO
* Lunit SCOPE IO pivotal in first phase III trial successfully showing the efficacy of immunotherapy + chemotherapy for NSCLC patients with EGFR or ALK mutation - published in theJournal of Clinical Oncology and accepted by ESMO for oral presentation SEOUL, South Korea, Oct. 24, 2023 /PRNewswir...
Phase Ib/II Results of Akeso's PD-1/CTLA-4 Bispecific Antibody for First-Line Treatment of Advanced Hepatocellular Carcinoma Published at 2023 ESMO
HONG KONG, Oct. 24, 2023 /PRNewswire/ -- Akeso announced poster presentation at the 2023 European Society for Medical Oncology (ESMO) Congress from its cadonilimab ( PD-1/CTLA-4 bispecific antibody) combined with lenvatinib for first-line treatment of Advanced Hepatocellular Carcinoma (HCC). The ...
Clinical Trial Application (IND) for BioRay Pharmaceutical's BR105 Injection Receives U.S. FDA Approval
SHANGHAI, Oct. 24, 2023 /PRNewswire/ -- BioRay Pharmaceutical Co., Ltd. (hereinafter referred to as "BioRay") announced onOctober 7th that its clinical trial application for BR105 Injection has received approval from the United States Food and Drug Administration (FDA). This marks BioRay's first ...
OBiO Announces Strategic Partnership with Refreshgene to Realize Commercialization of Gene Therapy Product
Visit OBiO at ESGCT 30th Annual Congress in Brussels SHANGHAI, Oct. 24, 2023 /PRNewswire/ -- OBiO Technology (Shanghai) Corp., Ltd. (OBiO, 688238.SH), a world leading contract development and manufacturing organization for cell and gene therapy, officially announced an agreement with Refreshgene...
Wingderm® Has Unveiled the Latest Research of Mesoskin for Mesotherapy Treatment at AMWC
BEIJING, Oct. 23, 2023 /PRNewswire/ -- On October 20-22, 2023, the Aesthetic & Anti-Aging Medicine World Congress (AMWC) was held inChengdu, China. A wide range of aesthetic plastic surgery products and technologies made their appearance at this event, Wingderm®'s Latest Research of Mesoskin for ...
Genesis MedTech initiates enrollment in its North American Early Feasibility Study using the J-Valve™ Transfemoral System for patients with severe aortic regurgitation
BURLINGAME, Calif., Oct. 23, 2023 /PRNewswire/ -- Genesis MedTech, a leading medical device company, today announced initiation of enrollment in its North American Early Feasibility Study using its dedicated TAVR system, J-Valve™ Transfemoral (TF) System. The procedure was successfully perform...
Certa Therapeutics' FT011 Granted US FDA Orphan Drug Designation for the Treatment of Systemic Sclerosis
MELBOURNE, Australia, Oct. 23, 2023 /PRNewswire/ -- Certa Therapeutics (Certa), a biotechnology company developing innovative precision therapies for patients with inflammatory and fibrotic diseases, today announces that the U.S. Food and Drug Administration (FDA) granted Orphan Drug Designation ...
WAT Medical Donates to the Canadian Red Cross and Peach Arch Hospital
VANCOUVER, BC, Oct. 23, 2023 /PRNewswire/ -- WAT Medical Enterprise Ltd. has
extended its support to two prominent institutions: the Canadian Red Cross and
Peach Arch Hospital, throughmonetary donations.
Week's Top Stories
Most Reposted
Wonder Raises USD 12 Million Venture Debt from HSBC Innovation Banking to Drive Growth and Expansion
[Picked up by 322 media titles]
2026-02-02 10:00AI adoption is widespread, but developer confidence is still catching up, Agoda report finds
[Picked up by 313 media titles]
2026-02-03 11:00Mastercard Launches Portfolio of Fleet Solutions in Asia Pacific
[Picked up by 310 media titles]
2026-02-04 09:00Colebrook Bosson Saunders Officially Launches Lana, A Circular Ergonomic Laptop Stand for the Hybrid Generation
[Picked up by 304 media titles]
2026-02-03 12:00Singapore Airshow 2026 Milestone Edition: 20 Years of Shaping the Aerospace Landscape as Asia-Pacific Drives Global Growth
[Picked up by 287 media titles]
2026-02-01 19:35