Medical/Pharmaceuticals
KAZIA MARKS DIPG AWARENESS DAY; NEW PRECLINICAL DATA IN DIPG TO BE PRESENTED AT ISPNO CONFERENCE
SYDNEY, May 17, 2022 /PRNewswire/ -- Kazia Therapeutics Limited (NASDAQ: KZIA; ASX: KZA), a late-stage, oncology-focused drug development company, marks 'DIPG Awareness Day' by recognizing the tireless work of global researchers in this disease, and by calling for an increased focus on childhood ...
GeneQuantum Healthcare's Two Next Generation Bioconjugate Drugs, GQ1005 and GQ1007, Have Been Approved for Clinical Trials in Australia
SUZHOU, China, May 15, 2022 /PRNewswire/ -- GeneQuantum Healthcare (Suzhou) Co., Ltd. (hereinafter referred to as "GeneQuantum"), a global innovative biotechnology company dedicated to the development of bioconjugate drugs, announced that two of the company's bioconjugate drug candidates have won...
Kintor Pharma Included in MSCI China Index
SUZHOU, China, May 15, 2022 /PRNewswire/ -- Kintor Pharmaceutical Limited ("Kintor Pharma," HKEX: 9939), a clinical-stage biotechnology company developing innovative small molecules and biological therapeutics, announced today that the company's stock has been included in the MSCI China Index, e...
Dizal Announces New Data from the Phase I/II Study of Golidocitnib in Refractory or Relapsed Peripheral T Cell Lymphoma Selected for Oral Presentation at 2022 EHA Annual Meeting
SHANGHAI, May 13, 2022 /PRNewswire/ -- Dizal (SSE: 688192) will present the data from an ongoing phase I/II study of golidocitnib, a selective JAK1 inhibitor, in refractory or relapsed peripheral T cell lymphoma (r/r PTCL) in oral session at the 27th European Hematology Association 2022 (EHA2022)...
Yiling Pharmaceutical: Lianhua Qingwen Approved in Nigeria
SHIJIAZHUANG, China, May 12, 2022 /PRNewswire/ -- Yiling Pharmaceutical disclosed Wednesday that it had received the medicine approval document for Lianhua Qingwen Capsules from the National Agency for Food and Drug Administration and Control (NAFDAC),Nigeria. The Product Category is Herbal and ...
Gracell Biotechnologies to Present Clinical Data on BCMA/CD19 Dual-targeting CAR-T GC012F in RRMM and B-NHL and CD19/CD7 Dual-directed Allogeneic CAR-T GC502 in B-ALL at EHA2022 Congress
SAN DIEGO, Calif., SUZHOU and SHANGHAI, China , May 12, 2022 /PRNewswire/ -- Gracell Biotechnologies Inc. ("Gracell" or the "Company", NASDAQ: GRCL), a global clinical-stage biopharmaceutical company dedicated to developing highly efficacious and affordable cell therapies for the treatment of can...
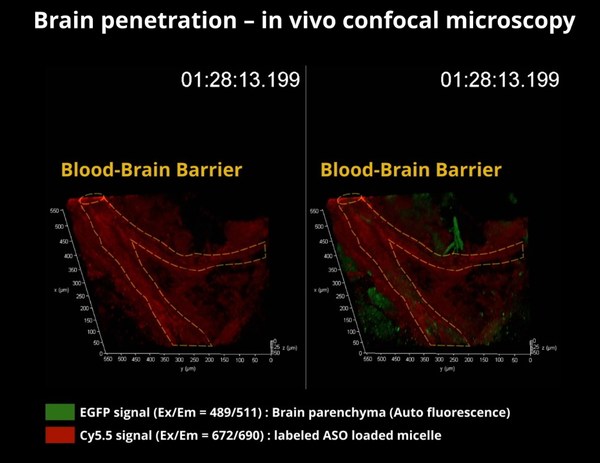
BIORCHESTRA Participated in Formulation and Delivery
BOSTON, May 12, 2022 /PRNewswire/ -- BIORCHESTRA is a leading RNA therapeutics
company that develops novel RNA-based therapeutics and drug delivery systems
for neurodegenerative diseases.
Formosa Pharmaceuticals and AimMax Therapeutics Announce Successful Top-Line Results from CPN-301 for the Treatment of Inflammation and Pain after Cataract Surgery
TAIPEI, May 12, 2022 /PRNewswire/ -- Formosa Pharmaceuticals, Inc. (TWO.6838
A novel universal antiviral drug introduced in Korea
- Hyundai Bioscience launches CP-COV03 Phase 2 clinical trials - "To be an innovative medicine like penicillin that led to victory in the bacterial war" - Significant changes to the 'vaccine-dependent' virus response system SEOUL, South Korea, May 12, 2022 /PRNewswire/ -- The birth of a universa...
Zhimeng Biopharma Announces Dosing of First Subject of Its Novel Antiepileptic Drug Candidate CB03 in First-in-Human Phase I Clinical Trial
SHANGHAI, May 11, 2022 /PRNewswire/ -- Shanghai Zhimeng Biopharma, Inc. ("Zhimeng"), announced dosing of the first participant in the US Phase I study in healthy subjects of its innovative small-molecule KCNQ2/3 selective opener (CB03), developed for the treatment of refractory epilepsy. CB03 is...
Asia's first molecular diagnostics company for women, INEX Innovate receives European CE-IVD Certification for its OvaCis(R) Rapid Test to detect cancer in ovarian cysts.
Revolutionary Ovarian Cancer Rapid Test Available Q4 2022 SINGAPORE, May 11, 2022 /PRNewswire/ -- Known as a pioneer in Asia's women's and fetal health industry,Singapore-based diagnostics developer, INEX Innovate has obtained a CE markfor its lead ovarian cancer product, the OvaCis® Rapid Test....
PharmAbcine to participate in Jefferies Global Healthcare Conference and BIO International Convention
DAEJEON, South Korea, May 11, 2022 /PRNewswire/ -- PharmAbcine Inc. (KOSDAQ: 208340ks), a clinical-stage biotech company focusing on the development of next-generation antibody therapeutics, announced today that Dr.Jin-San Yoo, Chairman and Chief Executive Officer of PharmAbcine is invited to par...
Bridge Biotherapeutics to Announce Updated Data from its Phase 1 Study of BBT-176 in Advanced Non-Small Cell Lung Cancer in an Oral Presentation at IASLC 2022 World Conference on Lung Cancer
* Data analysis to explore demonstrated anti-tumor activity of BBT-176, a fourth generation EGFR TKI, in advanced non-small cell lung cancer SEONGNAM, South Korea , May 10 /PRNewswire/ -- Bridge Biotherapeutics Inc. (KQ288330), a South Korean clinical-stage biotechnology company focused on deve...
Nature Medicine Publishes Results from an Investigator-Initiated Trial of CARsgen's CT041 Claudin18.2 CAR T Cells in Gastrointestinal Cancers
SHANGHAI, May 10, 2022 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, announces that the phase I trial interim results of an investigator-initiated tri...
Harbour BioMed to Present the Latest Progress of Next-Generation Anti-CTLA-4 Antibody HBM4003 at 2022 ASCO Annual Meeting
CAMBRIDGE, Mass. and ROTTERDAM, Netherlands and SUZHOU, China, May 10, 2022 /PRNewswire/ -- Harbour BioMed (the "Company", HKEX: 02142), a global biopharmaceutical company committed to the discovery, development, and commercialization of novel antibody therapeutics, today announced the Company w...
AffaMed Therapeutics Holds DEXTENZA Launch and Advisory Board Meeting in Macau
SHANGHAI, May 9, 2022 /PRNewswire/ -- AffaMed Therapeutics ("AffaMed"), a global clinical-stage biopharmaceutical company dedicated to developing and commercializing transformative pharmaceutical, digital and surgical products, held a DEXTENZA Launch and Advisory Board Meeting recently inMacau, f...
Formus Labs, a Pioneering Developer of Orthopedic Surgery Planning Technology, Hires Ex-Johnson & Johnson Executive, Polly Teevan, To Lead Global Commercial Strategy
Having managed a nearly $1 billion business in the world's largest orthopedic market, Teevan brings 20+ years of experience to help Formus improve patient lives AUCKLAND, New Zealand, May 10, 2022 /PRNewswire/ -- Formus Labs, creator of the world's first fully-automated 3D planner for joint repl...
iNtRON Biotechnology, Inc., Launches New Website
It aims to promote strategic collaborations in global while also highlights its many innovative advances and successful scientific ventures. BOSTON and SEOUL, South Korea, May 9, 2022 /PRNewswire/ -- The parent company of iNtODEWORLD, Inc., iNtRON Biotechnology, Inc., ("iNtRON" or "Company") is ...
AskGene Limited Announces Completion of USD 20 Million Series A Financing Led by Qiming Venture Partners and TF Capital
CAMARILLO, Calif. and NANJING, China, May 8, 2022 /PRNewswire/ -- AskGene Limited ("AskGene"), a clinical-stage biopharmaceutical company focusing on discovery and development of innovative biological drugs, today announced the completion ofUSD 20 million in Series A financing. The financing was ...
InxMed Raised $15 million in Series B+ Financing to Advance Innovative Therapies to Drug-resistant Cancers
NANJING, China, May 8, 2022 /PRNewswire/ -- InxMed Co., Ltd, a clinical-stage biotechnology company dedicated to developing innovative therapies targeting stroma microenvironment and drug resistance for hard-to-treat solid tumors, today announced that it had completed $15 million in Series B+ Fin...
Week's Top Stories
Most Reposted
Rocket Travel by Agoda Shares Revealing New Report and Showcases Solution to Transform Hotel Distribution
[Picked up by 314 media titles]
2024-11-21 10:30Rockwell Automation and Microsoft Deliver on a Shared Vision to Accelerate Industrial Transformation
[Picked up by 308 media titles]
2024-11-20 13:29Durabook and Parent Company, Twinhead International Corp., Celebrate 40 Years of Innovation in Computing Solutions
[Picked up by 301 media titles]
2024-11-20 16:30Travel loyalty programs to focus on offering personalized and flexible customer experiences in 2025
[Picked up by 298 media titles]
2024-11-19 10:42Philips and Edith Cowan University Australia Collaborate to Equip the Next Generation of Healthcare Professionals to leverage new technologies
[Picked up by 284 media titles]
2024-11-20 09:00