Medical/Pharmaceuticals
Tigermed Recognized with Frost & Sullivan 2021 China Clinical CRO Competitive Strategy Leadership Award
HANGZHOU, China, Nov. 29, 2021 /PRNewswire/ -- Tigermed, a leading provider of innovative clinical research solutions for biopharmaceutical and medical device industry, announced that it has been awarded the Frost & Sullivan 2021 China Contract Research Organization (CRO) Competitive Strategy Lea...
Tang Prize Laureates Reflect on the Breakthroughs Made Possible by Cytokine Research
TAIPEI, Nov. 27, 2021 /PRNewswire/ -- Following the inspiring opening speech, "Future Perspective of Cancer Immunotherapy," delivered by Nobel Prize and Tang Prize laureate Prof.Tasuku Honjo at the 14th Asia Pacific Federation of Pharmacologist Conference (APFP) onNovember 26, the 2020 Tang Prize...
IASO Biotheraputics' CT120 Granted Orphan Drug Designation by the U.S. FDA
SAN JOSE, Calif., NANJING, China and SHANGHAI, Nov. 25, 2021 /PRNewswire/ -- IASO Biotherapeutics (IASO Bio), a clinical-stage biopharmaceutical company focusing on discovering, developing, and manufacturing innovative medicine announcedon Oct 26, 2021 that the Office of Orphan Products Developme...
Biotron Drug Effective Against COVID-19 In Animals
* BIT225 administered orally significantly reduced viral load in the lungs and blood of animals challenged with SARS-CoV-2. * BIT225 protected against severe disease, indicated by the significant prevention of body weight loss in animals treated with BIT225 compared to non-treated controls. ...
Kintor Pharma Receives IND Clearance by NMPA for KX-826's Pivotal Study to Treat Male Alopecia Patients
SUZHOU, China, Nov. 24, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited ("Kintor Pharma", HKEX: 9939), a clinical-stage biotechnology company developing innovative small molecule and biological therapeutics, today announced that the IND application for the pivotal study (phase III stage) of p...
Neurophth Therapeutics Strengthens Leadership Team with Appointment of Su Zhang as Chief Financial Officer
SHANGHAI and SAN DIEGO, Nov. 24, 2021 /PRNewswire/ -- Neurophth Biotechnology Ltd., a fully-integrated genomic medicines company seeking to improve lives through the curative potential of gene therapies, today announced the recent appointment ofSu Zhang, M.B.A., as the Chief Financial Officer (CF...
GreenLight Biosciences and Samsung Biologics announce collaboration to build capacity for messenger RNA Vaccine Manufacturing
BOSTON and INCHEON, South Korea, Nov. 24, 2021 /PRNewswire/ -- GreenLight Biosciences (Nasdaq: ENVI), a biotechnology company dedicated to making ribonucleic acid (RNA) products affordable and accessible for human health and agriculture, and Environmental Impact Acquisition Corp, and Samsung Biol...
Next-generation Precision Medicine Company Allorion Therapeutics Completes Series A Financing Round
BOSTON, Nov. 24, 2021 /PRNewswire/ -- Allorion Therapeutics, a company focusing on next-generation precision medicine for oncology and autoimmune disease, today announced the completion of$40M Series A financing. This financing round is led by Qiming Venture Partners and participated by IDG Capit...
Amgen and Bushu Pharma Announce Expanded Supply Chain Partnership across Japan and Asia-Pacific Region
KAWAGOE, Japan, Nov. 24, 2021 /PRNewswire/ -- Bushu Pharmaceuticals Ltd. ("Bushu Pharma") has agreed on an expanded partnership with Amgen Inc. and Amgen K.K. to provide its "Gateway toAsia" service to four countries across the Asia-Pacific region as Amgen's contract manufacturing organization (C...
World's First|Aopeng Medical Completes Clinical Trial of Robotic-Assisted Peripheral artery Intervention of iliac artery in human
SHANGHAI, Nov. 23, 2021 /PRNewswire/ -- On November , 2021, Aopeng Medical's ALLVAS™ Endovascular Intervention Surgical Robot, successfully completed the first-in-human clinical evaluation of robot-assisted peripheral artery intervention of iliac artery in the Department of Vascular Surgery at Sh...
Everest Medicines Announces Inclusion in the MSCI Global Small Cap Indexes - MSCI China Index
SHANGHAI, Nov. 23, 2021 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK, "Everest" or the "Company"), a biopharmaceutical company focused on developing and commercializing transformative pharmaceutical products that address critical unmet medical needs for patients inAsia, today announced that th...
Gracell Biotechnologies Announces Senior Management Team Share Purchase Plan
SUZHOU, China and PALO ALTO, Calif., Nov. 23, 2021 /PRNewswire/ -- Gracell Biotechnologies Inc. (NASDAQ: GRCL) ("Gracell" or the "Company"), a global clinical-stage biopharmaceutical company dedicated to developing highly efficacious and affordable cell therapies for the treatment of cancer, toda...
Neurophth Raised Over $60 Million USD in Series-C Financing to Advance Gene Therapies
SAN DIEGO, Nov. 23, 2021 /PRNewswire/ -- Neurophth Therapeutics Ltd., ("Neurophth"), a fully integrated genomic medicines company dedicated on seeking to improve lives through the curative potential of gene therapies, today announced the closing of over$60 million USD in Series-C financing with a...
Qilian International Holding Group Limited Announces Inclusion of Gan Di Xin® and Qilian Shan® Oxytetracycline into List for Development of Leading Enterprises with Large Varieties and Brands of Pharmaceuticals of Gansu Province
JIUQUAN, China, Nov. 23, 2021 /PRNewswire/ -- Qilian International Holding Group Limited (Nasdaq: QLI) (the "Company"), aChina-based pharmaceutical and chemical products manufacturer, today announced that Gan Di Xin® and Qilian Shan® Oxytetracycline, products of the Company's subsidiary, Gansu Qi...
Bionomics Files Registration Statement for Proposed Initial Public Offering in the United States
ADELAIDE, Australia, Nov. 23, 2021 /PRNewswire/ -- Bionomics Limited (ASX:BNO, OTCQB:BNOEF) (Bionomics or Company), a clinical-stage biopharmaceutical company, today announced the public filing of a registration statement on Form F-1 with the U.S. Securities and Exchange Commission (the "SEC") in...
Simcere and Shanghai Institute of Materia Medica Enter a Collaboration to Jointly Develop Next-Gen COVID-19 Oral Antiviral Treatments
NANJING, China, Nov. 22, 2021 /PRNewswire/ -- On November 17, 2021, Simcere Pharmaceutical Group Limited (2096.HK) announced that the company has reached collaboration agreement with the Shanghai Institute ofMateria Medica (SIMM), Chinese Academy of Sciences (CAS), to develop novel antiviral drug...
Trajan Group acquires Axel Semrau GmbH
MELBOURNE, Australia, Nov. 22, 2021 /PRNewswire/ -- Global analytical science and device company Trajan Group Holdings Limited (ASX: TRJ) (Trajan or the Company) has completed the acquisition of Axel Semrau GmbH & Co. KG and Semrau Immobilien GmbH & Co. KG, the private business and properties of ...
Arctic Vision Announces First Patient Dosed in Phase III Study of ARVN001 for Treatment of Macular Edema Associated with Uveitis (UME)
* First-in-class SCS Microinjection therapy * First clinical registration study and potentially the first approved drug in China targeting macular edema associated with uveitis * U.S. FDA approved for treatment of macular edema associated with uveitis SHANGHAI, Nov. 22, 2021 /PRNewswire/ -- A...
Ascentage Pharma's Olverembatinib Granted Orphan Designation by the European Commission for the Treatment of Chronic Myeloid Leukemia
SUZHOU, China, and ROCKVILLE, MD., Nov. 22, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that the European Commission (EC) has granted the...
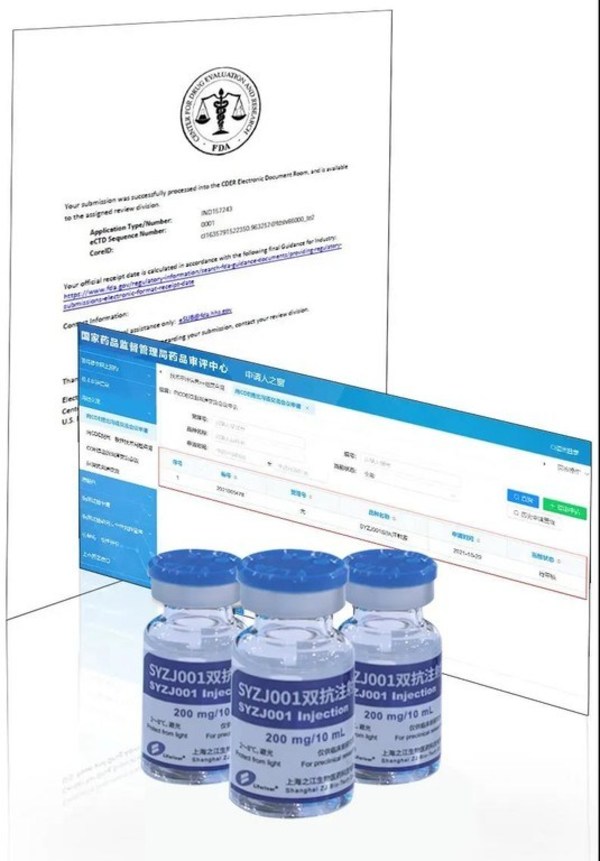
Bispecific antibody drug against COVID-19 jointly developed by Shanghai ZJ Bio-Tech and Sanyou Biopharmaceuticals appearing at China International Import Expo (CIIE)
SHANGHAI, Nov. 22, 2021 /PRNewswire/ -- At the opening ceremony of the 4th China International Import Expo (CIIE) held inShanghai on November 6, 2021, SYZJ001, a bispecific antibody drug against COVID-19 jointly developed by Shanghai ZJ Bio-Tech Co., Ltd. and Sanyou Biopharmaceuticals (Shanghai) ...
Week's Top Stories
Most Reposted
2024 Malaysia E-Commerce Product Selection Expo Attracts International Delegations
[Picked up by 307 media titles]
2024-12-02 12:06QDX Co-Founder Giuseppe Barca Awarded Prestigious Gordon Bell Prize, Pioneering Advancements in Drug Discovery Technology
[Picked up by 303 media titles]
2024-11-26 18:49ITAP 2024 sets the stage for a more connected advanced factory ecosystem with focus on AI, advanced robotics and sustainability
[Picked up by 298 media titles]
2024-11-28 17:24Southeast Asian Consumers Raise Online Security Concerns, New GSMA Survey Shows
[Picked up by 296 media titles]
2024-11-26 13:00Mentech at COP29: Showing the Eco-friendly Lifestyle with Technological Innovation
[Picked up by 284 media titles]
2024-11-29 20:33