Medical/Pharmaceuticals
Travecta Therapeutics Appoints Charles S. Ryan as President and Chief Executive Officer
SINGAPORE, June 9, 2021 /PRNewswire/ -- Travecta Therapeutics, Pte Ltd., a preclinical stage biopharmaceutical company pioneering a portfolio of product candidates engineered to cross the blood-brain-barrier, today announced the appointment ofCharles S. Ryan, JD, PhD as President and Chief Execut...
China Pharma to Launch Highly Purified NMN+PQQ Product
HAIKOU, China, June 9, 2021 /PRNewswire/ -- China Pharma Holdings, Inc. (NYSE American: CPHI) ("China Pharma," the "Company" or "We"), a specialty pharmaceutical company, today announced plans to launch a highly purified NMN+PQQ product following the recent successful completion of a pilot scale ...
Study of Paxalisib in Primary CNS Lymphoma at Dana-Farber Cancer Institute Enrols First Patient
SYDNEY, June 7, 2021 /PRNewswire/ -- Kazia Therapeutics Limited (NASDAQ: KZIA; ASX: KZA), an oncology-focused drug development company, is pleased to announce that a phase II study of Kazia's investigational new drug, paxalisib, in primary CNS lymphoma has been initiated at Dana-Farber Cancer Ins...
Yingli Pharma Announces presentations on the Phase 1 trials of linperlisib, a PI3Kδ selective inhibitor, and YL-13027, an oral TGFβR1 inhibitor, at the American Society for Clinical Oncology 2021 Annual Meeting
Clinical trial data will be updated in three presentations on lead drug candidates * Linperlisib, a potent oral PI3Kδ inhibitor, demonstrated robust clinical activity with 70% ORR in relapsed or refractory Peripheral T-cell Lymphoma * Linperlisib is demonstrated to be well-tolerated in a 78 p...
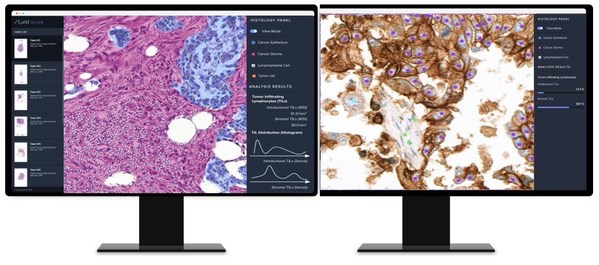
AI-based Analysis of Cancer Tissue Predicts Response to Immunotherapy--Findings to Be Presented at ASCO 2021
* Lunit to present four abstracts including one in a 'Poster Discussion Session' * AI-based tissue analysis platform 'Lunit SCOPE' to be launched within the second half of 2021: "We aim to make our AI the new standard for cancer treatment" SEOUL, Korea, June 3, 2021 /PRNewswire/ -- A new find...
China NMPA Approves MicuRx's Contezolid for Treatment of Drug-Resistant Bacterial Infection
SHANGHAI, June 3, 2021 /PRNewswire/ -- MicuRx Pharmaceutical Co., Ltd. (MicuRx), a leading biopharmaceutical company focused on the discovery, development and commercialization of antibacterial agents against drug-resistant bacterial infection, announced that China National Medical Products Admi...
Registration Open for the 4th China Medical Devices Design & Entrepreneurship Competition 2021
SUZHOU, China, June 2, 2021 /PRNewswire/ -- In order to boost the continuous innovation and development ofChina's medical device industry, deepen the cooperation among the medical device industry, universities, research institutions, users, capital sector and regulators in all aspects, and stimu...
Cell Care Joins the Generate Life Sciences Family to Create a Global Platform
The acquisition provides the companies with shared scientific knowledge and resources leading to a stronger foundation for advancing newborn stem cell banking and research MELBOURNE, Australia, June 3, 2021 /PRNewswire/ -- Cell Care, Australia's leading cord blood bank, today announced it has be...
GenScript Biotech and Ligand Pharmaceuticals Enter into Global OmniAb® Licensing Agreement
NANJING, China and SAN DIEGO, June 2, 2021 /PRNewswire/ -- GenScript Biotech Corporation (HKG: 1548) along with its subsidiary, GenScript ProBio, and Ligand Pharmaceuticals Incorporated (NASDAQ: LGND) announced today that they have entered into a strategic licensing agreement for Ligand's OmniAb®...
Shanghai Asclepius Meditec and International Department of Central Committee of CPC Collaborate to Provide Emergency Support to Fight COVID-19 in Nepal
SHANGHAI, June 2, 2021 /PRNewswire/ -- The President of Asclepius Meditec – the
company behind the innovativeHydrogen Oxygen Generator with Nebulizer
PharmAbcine to Present at the BIO Digital International Convention 2021
DAEJEON, South Korea, June 2, 2021 /PRNewswire/ -- PharmAbcine Inc. (KOSDAQ: 208340ks), a clinical-stage biotech company focusing on the development of antibody therapeutics, announced today that the company will participate in BIO Digital International Convention. The company will participate in...
Samsung Biologics and KAHR Medical Announce Development and Manufacturing Agreement for Cancer Immunotherapy Drug Candidate
INCHEON, South Korea, June 2, 2021 /PRNewswire/ -- Samsung Biologics (KRX: 207940.KS), the world's leading contract development and manufacturing organization, signed a strategic partnership with KAHR Medical Ltd., a cancer immunotherapy company developing novel multifunctional immune-recruitment...
ImmVira will present the U.S. Clinical Phase I Study Results of MVR-T3011 via Intratumoral Administration at ASCO 2021
SHENZHEN, China, June 2, 2021 /PRNewswire/ -- ImmVira will present results from
its clinical Phase I study of MVR-T3011 via intratumoral (IT) administration at
the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting on June 4-
8, 2021 for the first time.
Therabody™ Announces Partnership With World-Renowned Footballer Cristiano Ronaldo
The Company Releases its Largest Global Ad Campaign since its Founding to Amplify the Importance of Recovery LOS ANGELES, June 2, 2021 /PRNewswire/ -- Therabody, the global leader in tech wellness and the creators of theTheragun®, announced today professional footballerCristiano Ronaldo as the c...
Bridge Biotherapeutics Announces the Initiation of the Proof of Mechanism Study for BBT-401 in Patients with Ulcerative Colitis; Rectal Delivery of Drug to Target Site
* The new proof of mechanism trial in ulcerative colitis had its first patient dosed inNew Zealand * In parallel with the oral-administered, proof of clinical principle study, the new proof of mechanism study will examine the efficacy of BBT-401 when delivered directly to the target organ via...
Eucure Biopharma to Present Findings From Anti-CD40 and Anti-CTLA-4 mAb Clinical Trials at the 2021 ASCO Meeting
BEIJING and BOSTON, June 1, 2021 /PRNewswire/ -- Eucure Biopharma will present findings from two Phase I clinical trials at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, held fromJune 4th to 8th. The first trial (poster #2580; NCT04481009) is designed to assess the safety...
Daewoong Pharmaceutical Publishes Results of First Clinical Study of SGLT-2 Inhibitor 'Enavogliflozin' for Canine Diabetes
SEOUL, South Korea, June 1, 2021 /PRNewswire/ -- Daewoong Pharmaceutical (CEO Sengho Jeon) announced that the results of an investigator-initiated clinical study on the effect of Enavogliflozin, originally developed as Type II Diabetes Treatment forhumans, on treating canine diabetes were publish...
GenScript ProBio and Neoletix signed a letter of intent on clinical and commercial production of recombinant human coagulation factor VIII
NANJING, China, June 1, 2021 /PRNewswire/ -- On June 1st 2021, GenScript ProBio and Neoletix (Beijing) Biotechnology Co., Ltd. (hereinafter referred to as Neoletix) signed a letter of intent on cooperation for clinical and commercial production of human coagulation factor VIII. GenScript ProBio s...
Neurophth Therapeutics and Hopstem Biotechnology Announce Strategic Partnership to Develop Human Induced Pluripotent Stem Cell-Derived Therapies for the Treatment of Ocular Diseases
HOUSTON and SAN DIEGO, June 1, 2021 /PRNewswire/ -- Neurophth Biotechnology Ltd., a fully-integrated genetic medicines company developing AAV-mediated gene therapies for the treatment of ocular diseases, and Hopstem Biotechnology, the leading human induced pluripotent stem cell (hiPSC) and neural...
Positive FDA meetings enable Neuren to proceed with INDs for three Phase 2 trials
Highlights: * Clear and constructive guidance received from pre-IND meetings with the FDA Office of Neuroscience for Phase 2 clinical trials of NNZ-2591 in Phelan-McDermid, Angelman andPitt Hopkins syndromes * Positive outcome is an important milestone that enables Neuren to proceed with IND...
Week's Top Stories
Most Reposted
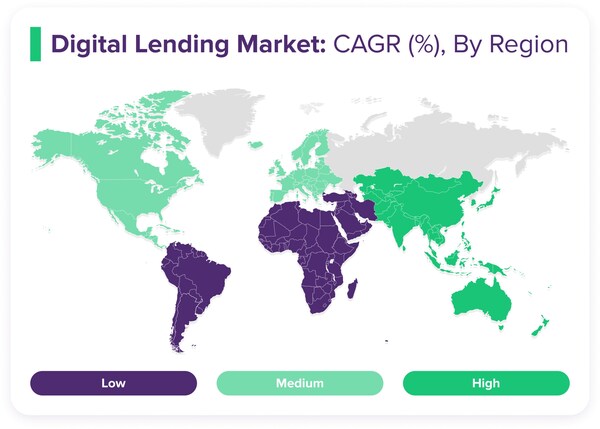
ROSHI Unveils Comprehensive Report on the Future of Digital Lending, Highlighting Global Trends for 2025 and Beyond
[Picked up by 317 media titles]
2024-10-02 10:03From Boomers to Zoomers: how retailers and eCommerce firms can boost sales and loyalty
[Picked up by 305 media titles]
2024-09-30 13:00Matterport Fall 2024 Release: Insights Meets Imagination elevates the platform with generative AI and more
[Picked up by 305 media titles]
2024-10-02 09:00Graid Technology and INFINITIX Turn Your SuperPOD into a Real Business Opportunity at Tech Week Singapore
[Picked up by 293 media titles]
2024-10-04 11:00MOAJ Holding invests $30M in Joint Venture with MediSun Energy to Tackle Water Scarcity and Brine Challenges in Saudi Arabia
[Picked up by 293 media titles]
2024-09-30 09:00