Medical/Pharmaceuticals
Introduce the World's First Biomedical Application Mask that Revolutionizes Face Mask to Resist Aerosol Transmission
TAIPEI, May 31, 2021 /PRNewswire/ -- Taer Innovation has won design awards including Good Design award from 2012 to 2014 Golden Pin Design award, Taiwan Excellence award in 2014 and Computex d&I award in 2014 for their products.The companyfocuses on medical innovation and technology. The company'...
Samsung Biologics to add mRNA vaccine drug substance manufacturing suite to expand portfolio of services
* Samsung Biologics, a global leading CDMO, will add mRNA vaccine DS production capability to its existing facility by the first half of 2022 * The addition will enable Samsung Biologics to provide end-to-end mRNA vaccine manufacturing services from bulk drug substance to aseptic fill/finish ...
Dr. Seungpil Yu will hold a retirement ceremony and conclude his 46-year term as a pharmaceutical executive.
SEOUL, South Korea, May 28, 2021 /PRNewswire/ -- Dr. Seungpil Yu is a living witness to the long history of Yuyu Pharma, which celebrated its 80th anniversary this year, having laid the foundation for Yuyu Pharma to continue to thrive well into its 100th year. While in the United States, Dr. Yu r...
Revolutionary X-ray Technology From Australia to Transform American Airport Security
SeaTac chosen for Micro-X Inc's USA headquarters Official Opening at 10 am PDT, Friday May 28 855 S 192nd Street, Suite B600, Seattle, WA 98148 With: * Congressman Adam Smith, chair of the House Armed Services Committee * Brigadier Hugh Meggitt representing Australian Defence Staff, Embassy o...
Samsung Biologics Adds Four Global ISO Certifications For BCMS, Energy, Health & Safety, and Environmental Management
* Expanding upon its existing ISO22301 certification, Samsung Biologics is now fully certified with Business Continuity Management System for Security and Resilience across all business areas * Samsung Biologics adds ISO50001, ISO45001, ISO14001 for Energy Management, Occupational Health and ...
AffaMed Therapeutics Announces Approval for the Phase IIb Clinical Study of AM006 in China for the Treatment of Parkinson's Disease
SHANGHAI, May 27, 2021 /PRNewswire/ -- AffaMed Therapeutics ("AffaMed"), a global clinical stage biopharmaceutical company dedicated to addressing critical unmet medical needs in ophthalmic, neurological and psychiatric disorders, today announced thatChina's National Medical Products Administrati...
Daewoong, first-in-class PRS-inhibitor for Idiopathic Pulmonary Fibrosis(IPF) shows promising result in early stage clinical test
* We confirmed the safety of 'DWN12088' and secured grounds for establishing treatment dosage * Confirming the potential as a treatment for IPF and planning to apply for Ph.2 within 2021 * 2nd ODD granted as treatment for systemic sclerosis from the US FDA SEOUL, South Korea, May 27, 2021...
Menarini Silicon Biosystems launches new breakthrough DEPArray™ PLUS image-based cell sorter to isolate rare cells with single cell precision
DEPArray™ PLUS – the new automated and versatile platform to investigate pre-selected rare cell populations from various samples with 100% purity, now enhanced with 9 fluorescent channels and remarkably improved user experience BOLOGNA, Italy and HUNTINGDON VALLEY, Pa., May 27, 2021 /PRNewswire/ ...
Foresee Pharmaceuticals Announces FDA Approval of CAMCEVI® for the Treatment of Advanced Prostate Cancer; Accord BioPharma to Head the U.S. Commercialization
TAIPEI, May 26, 2021 /PRNewswire/ -- Foresee Pharmaceuticals (6576.TWO), ("Foresee") announced today that the U.S. Food and Drug Administration (FDA) has approved the New Drug Application (NDA) for CAMCEVI® 42 mg, a ready-to-use 6-month subcutaneous depot formulation of leuprolide mesylate, as a ...
TransThera announces collaboration with Roche to evaluate TT-00420 as part of a novel combination to treat GI cancers in China
NANJING, China, May 26, 2021 /PRNewswire/ -- TransThera Biosciences Co. Ltd. ( "TransThera" ), a clinical stage biotechnology company dedicated to developing innovative therapeutics across oncology, cardiovascular, and inflammatory diseases with major unmet medical needs globally and inChina, to...
Gracell Biotechnologies Hosting Key Opinion Leader Webinar on High Risk Multiple Myeloma, Treatment Challenges and Dual-Targeting CAR-T with Overnight Manufacturing - a Novel Approach for Treatment of Multiple Myeloma
SUZHOU, China and PALO ALTO, Calif., May 26, 2021 /PRNewswire/ -- Gracell Biotechnologies Inc. (NASDAQ: GRCL) ("Gracell"), a global clinical-stage biopharmaceutical company dedicated to discovering and developing highly efficacious and affordable cell therapies for the treatment of cancer, today ...
IT Tech Packaging, Inc. Officially Obtains FDA Approval for Surgical Mask Products
BAODING, China, May 26, 2021 /PRNewswire/ -- IT Tech Packaging, Inc. (NYSE AMERICAN: ITP) ("IT Tech Packaging" or the "Company"), a leading manufacturer and distributor of diversified paper products inNorth China, today announced that the Company has officially obtained approval for surgical mask...
Prosit Sole Biotechnology Initiates First-In-Human Clinical Trial of Novel Interferon Lambda Chimera in US
BALTIMORE, May 26, 2021 /PRNewswire/ -- Prosit Sole Biotechnology, a clinical-stage biotech company developing novel protein therapeutics, today announces that it has initiated the first-in-human ("FIH") Phase I clinical trial of PSP001 in US. PSP001 is a novel, long acting and potent interferon ...
I Peace's cell manufacturing facility "Peace Engine Kyoto" receives third-party certification as US cGMP compliant: the facility now meets the standard for both the US and Japanese markets
PALO ALTO, Calif., May 25, 2021 /PRNewswire/ -- I Peace, Inc. (CEO: Koji Tanabe ), aPalo Alto-based biotech start-up focusing on Nobel Prize-winning technology "induced pluripotent stem cells (iPSCs)" received a third-party certification as US FDA cGMP compliant for its manufacturing facility "Pea...
Procyrion Announces Successful First-in-Human Cases in Cardiorenal Syndrome (CRS) Patients with Aortix™ Percutaneous Mechanical Circulatory Support Device
Pilot CRS Study to Evaluate Performance of Novel Aortix device in Australia and the U.S. HOUSTON, May 25, 2021 /PRNewswire/ -- Procyrion, Inc., a medical device company dedicated to improving outcomes for patients with cardiac and renal impairment, announced today successful treatment of the fir...
Concord Medical Receives Notification from NYSE Regarding Delayed Filing of 2020 Annual Report
BEIJING, May 25, 2021 /PRNewswire/ -- Concord Medical Services Holdings Limited ("Concord Medical" or the "Company") (NYSE: CCM), a healthcare provider specializing in cancer care, research and prevention by operating a network of medically advanced comprehensive cancer hospitals and standalone r...
Singapore-based HeMo Bioengineering Receives China's NMPA Approval for flagship Afentta(TM) Aspiration Catheter
SINGAPORE, May 24, 2021 /PRNewswire/ -- HeMo Bioengineering Ltd ("HeMo"), a Singapore-based medical device company with a focus on treating stroke patients, is pleased to announce that its Afentta™ intracranial thrombectomy aspiration catheter – a product developed byHeMo's branch in China – has ...
Two Products of Zhaoke Ophthalmology (6622.HK) Passed The On-Site Inspection for Drug Registration of The National Medical Products Administration and The GMP Compliance Inspection of The Guangdong Medical Products Administration
HONG KONG, May 24, 2021 /PRNewswire/ -- Zhaoke Ophthalmology Limited ("Zhaoke Ophthalmology" or the "Company"; stock code: 6622), an ophthalmic pharmaceutical company dedicated to the research, development and commercialization of therapies that address significant unmet medical needs, announc...
Genesis MedTech Group Raises Significant Growth Investment in Latest Round of Series B Financing
SINGAPORE, May 23, 2021 /PRNewswire/ -- Genesis MedTech Group ("Genesis"), a leading medical device company, today announced it has completed its Series B round, raising significant growth financing. General Atlantic, a leading global growth equity firm, led the funding with participation from CI...
A 'Game Changer' COVID-19 drug from Korea was introduced in a world class academic journal
SEOUL South Korea, May 23, 2021 /PRNewswire/ -- Hyundai Bioscience (KOSDAQ 048410) announced on the 24th that CNPharm, its major shareholding bio tech company, published an article containing the research results of CP-COV03, a Niclosamide-based oral treatment for COVID-19 based on its proprietar...
Week's Top Stories
Most Reposted
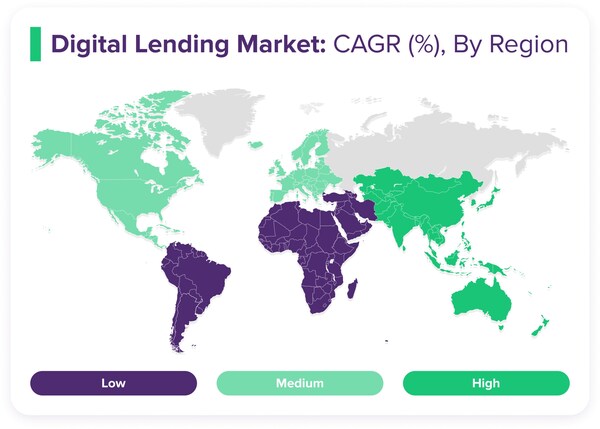
ROSHI Unveils Comprehensive Report on the Future of Digital Lending, Highlighting Global Trends for 2025 and Beyond
[Picked up by 317 media titles]
2024-10-02 10:03Matterport Fall 2024 Release: Insights Meets Imagination elevates the platform with generative AI and more
[Picked up by 305 media titles]
2024-10-02 09:00From Boomers to Zoomers: how retailers and eCommerce firms can boost sales and loyalty
[Picked up by 305 media titles]
2024-09-30 13:00MOAJ Holding invests $30M in Joint Venture with MediSun Energy to Tackle Water Scarcity and Brine Challenges in Saudi Arabia
[Picked up by 293 media titles]
2024-09-30 09:00Graid Technology and INFINITIX Turn Your SuperPOD into a Real Business Opportunity at Tech Week Singapore
[Picked up by 293 media titles]
2024-10-04 11:00