Pharmaceuticals
Ractigen Announces U.S. FDA Rare Pediatric Disease Designation (RPDD) Granted to RAG-18 for the treatment of Duchenne Muscular Dystrophy
SUZHOU, China, July 25, 2024 /PRNewswire/ -- Ractigen Therapeutics, a pioneering developer of small activating RNA (saRNA) therapeutics, today announced that the U.S. Food and Drug Administration (FDA) has granted Rare Pediatric Disease Designation (RPDD) to RAG-18, one of the company's lead saRN...
IASO Bio Announces NMPA Approval of IND Application for IASO-782 for Treatment of New Indication -- Systemic Lupus Erythematosus (SLE)
SHANGHAI, NANJING, China and SAN JOSE, Calif. , July 25, 2024 /PRNewswire/ -- IASO Biotechnology ("IASO Bio"), a biopharmaceutical company dedicated to discovering, developing, manufacturing and commercializing innovative cell therapy and antibody products, hereby announces thatthe investigation...
Full-Life Technologies Announces the Appointment of Edward (Ted) Myles to Its Board of Directors
HEIDELBERG, Germany and GEMBLOUX, Belgium and SHANGHAI, China, July 21, 2024 /PRNewswire/ -- Full-Life Technologies, a fully integrated global radiotherapeutics company, today announcedthe appointment of Ted Myles to Full-Life's Board of Directors. Mr. Myles will serve as chairman of the audit c...
Treatment of Type 2 diabetes with Chiglitazar combined with Metformin Approved for listing by the National Medical Products Administration
SHENZHEN, China, July 19, 2024 /PRNewswire/ -- On July 19, Shenzhen Chipscreen Biosciences Co., Ltd. (hereinafter referred to as "Chipscreen Biosciences") announced that it has received the Drug Registration Certificate approved and issued by the National Medical Products Administration (NMPA) fo...
Harbour BioMed Announces Positive Profit Alert for 2024 Interim Results
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, July 18, 2024 /PRNewswire/ -- Harbour BioMed (the "Company"; HKEX: 02142), a global biopharmaceutical company committed to the discovery, development, and commercialization of novel antibody therapeutics focusing on oncology and immunol...
ACROBiosystems Launches GMP Brand Resilient Supply at ISSCR to Accelerate Cell and Gene Therapy Development
NEWARK, Del., July 18, 2024 /PRNewswire/ -- At the recent International Society for Stem Cell Research (ISSCR) Annual Meeting inHamburg, ACROBiosystems announced its new GMP-centered brand, Resilient Supply, with the mission of accelerating the advancement of cell and gene therapy (CGT) developme...
Assessment of supply chain risk key to improving medicine access
A four-year research project by INSEAD and five other institutions sheds light on how understandingmedical criticality, supply chain risk and their interactions could help us better address drug shortages. FONTAINEBLEAU, France, SINGAPORE and SAN FRANCISCO, July 18, 2024 /PRNewswire/ -- Drug s...
Duoning showcases its comprehensive bioprocess solutions at Interphex Korea 2024
SHANGHAI, July 18, 2024 /PRNewswire/ -- Duoning Biotechnology Group ("Duoning"), a leading one-stop bioprocess solution provider, announced it has attended at Interphex Korea 2024 to showcase its total bioprocess solutions for the preparation of diverse biological products. Interphex 2024 provide...
MicuRx Presents Innovative Antibiotic Research Results at the 7th World Bronchiectasis Conference
* MicuRx showcased the latest research progress on their pipeline products, MRX-5 and MRX-8, at the 7th World Bronchiectasis Conference * Three significant study results further support the potential of MRX-5 and MRX-8 in treating Nontuberculous Mycobacteria (NTM) lung disease and Pseudomonas...
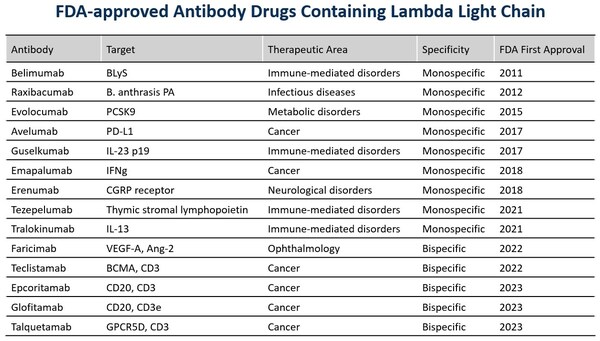
Increasing Possibility of Success by Expanded Antibody Repertoires with Lambda Light Chain - Immunocan Launches New Mouse Strain of Fully Human Antibody Platform: ImmuMab® HKL Mouse
BOSTON, July 17, 2024 /PRNewswire/ -- Immunocan announced the successful construction of new strain of fully human antibody platform: ImmuMab® HKL mouse. This new mouse was created by in-situ replacing the variable region of Immunoglobulin genes of mouse by its counterparts from human. With Lambd...
111, Inc. to Participate in Fireside Chat with Water Tower Research on July 18, 2024
SHANGHAIJuly 17, 2024 /PRNewswire/ -- 111, Inc. ("111" or the "Company") (NASDAQ: YI), a leading tech-enabled healthcare platform company committed to reshaping the value chain of healthcare industry by digitally empowering the upstream and downstream in China, today announced that it will partic...
GCCL Completes Proactive Development of Biosimilar Clinical Trial Analysis Methods, Providing Customized Analysis Services through Enhanced R&D Capabilities
* Newly established R&D division this year is expected to provide full-scale in-house R&D in addition to clinical sample analysis * Including blockbuster drugs such as 'Keytruda' and 'Yervoy', GCCL offers tailored clinical trial analysis services for biosimilar development YONGIN, South Korea...
Evolving DNA-Encoded Library Technology and Its Application for Innovative Drug Discovery, Upcoming Webinar Hosted by HitGen and Xtalks
CHENGDU, China, July 16, 2024 /PRNewswire/ -- "Evolving DNA-Encoded Library Technology and Its Application for Innovative Drug Discovery" webinar will be jointly hosted by HitGen Inc. and Xtalks onWednesday July 17, 2024, at 2pm EDT ( 11am PDT). Alex Shaginian, PhD, Vice President of Business Deve...
FDA Grants Orphan Drug Designation to 7MW3711
SHANGHAI, July 16, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovation-driven biopharmaceutical company with entire industry chain, announced that its self-developed novel B7-H3-targeting ADC (R&D code: 7MW3711) has been granted Orphan Drug Designation (ODD) by the U.S. Food and Drug Adminis...
Sanyou Bio Collaborates on ADC Drug Development Entering Phase I Clinical Trials
SHANGHAI, July 16, 2024 /PRNewswire/ -- Sanyou Biopharmaceuticals (Shanghai) Co., Ltd. (hereinafter referred to as "Sanyou Bio") has recently announced a significant milestone in collaboration with Hangzhou Zhongmei Huadong Pharmaceutical Co., Ltd., a wholly-owned subsidiary of Huadong Medicine C...
Mabwell Receives NMPA Approval for Clinical Trial of Novel Nectin-4 Targeting ADC in TNBC
SHANGHAI, July 15, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, announced its novel Nectin-4 targeting ADC (R&D code: 9MW2821) has been approved by the NMPA to enter Phase II clinical trial as monotherapy or in combination with a PD...
HanAll Biopharma Appoints Christopher W Slavinsky as Chief Business Development and Legal Officer
ROCKVILLE, Md. and SEOUL, South Korea, July 15, 2024 /PRNewswire/ -- HanAll
Biopharma (KRX: 009420.KS) announced today the appointment ofChristopher W.
Slavinsky as Chief Business Development and Legal Officer.
First Patient Dosed in Phase I Clinical Trial of YOLT-201
SHANGHAI, July 11, 2024 /PRNewswire/ -- YolTech Therapeutics, a biotech company developing in vivo gene editing therapies to treat rare genetic diseases, announced today the successful enrollment of the first patient in the Phase I clinical trial of YOLT-201, its independently developed in vivo g...
Sanyou Bio and BioGeometry join forces to create a next-generation Antibody Drug Discovery Platform driven by AIGC
SHANGHAI, July 11, 2024 /PRNewswire/ -- Recently, BioGeometry and Sanyou Bio jointly announced the signing of a strategic partnership agreement. BioGeometry is a digital biology pioneer company that specializes in AI-driven protein design and R&D platform. Sanyou Bio is a world-leading high-tech...
ADM Korea Announces Niclosamide-based Metabolic Anticancer Drug's First Clinical Trial Target as 'Prostate Cancer Patients Resistant to Hormone Therapy'
* IND for clinical study for combination therapy with hormone therapy in prostate cancer patients to be submitted in August, 2024. * Niclosamide-based metabolic anticancer drug aims to block signaling pathways that allow cancer cells to evade anticancer effects, potentially solving the issue ...
Week's Top Stories
Most Reposted
Agoda Launches Free Global eSIMs for VIP Diamond Members
[Picked up by 322 media titles]
2026-02-10 14:00Ascentium Acquires Clara, Expanding into the Abu Dhabi Global Market (ADGM) and Strengthening its Middle East Presence
[Picked up by 310 media titles]
2026-02-12 14:00Blackpanda Japan Announces Strategic Partnership with SoftBank to Strengthen Cyber Incident Response in Japan
[Picked up by 299 media titles]
2026-02-10 13:31Rockwell Automation Strengthens Industrial Cybersecurity with New Security Operations Center in Singapore
[Picked up by 299 media titles]
2026-02-09 10:00Carro unveils quirky generative AI ad campaign highlighting its 'Surprisingly Short' AI-enabled car-selling process
[Picked up by 296 media titles]
2026-02-11 11:00