Pharmaceuticals
Actinogen announces achievement of clinically and statistically significant superiority of Xanamem® over placebo on depression in XanaCIDD phase 2a trial
There was a clinically meaningful and persistent improvement depression measured by the key secondary endpoint of MADRS.[1] The primary endpoint of superiority to placebo in a cognitive "attention composite" of three Cogstate computerized tests was not met with large improvements seen in both Xan...
IASO Bio Receives U.S. FDA Approval of Investigational New Drug Application for Equecabtagene Autoleucel for Two New Autoimmune Disease Indications
SHANGHAI, NANJING, China and SAN JOSE, Calif., Aug. 12, 2024 /PRNewswire/ -- IASO Biotherapeutics ("IASO Bio"), a biopharmaceutical company dedicated to discovering, developing, manufacturing and commercializing innovative cell therapy and antibody products, today announced that the investigation...
T-MAXIMUM PHARMACEUTICAL Announces Latest Clinical Advances in Allogeneic CAR-T Cell Therapy Breakthrough for Solid Tumors
Summary * T-Maximum Pharmaceutical develops allogeneic CAR-T therapies with a unique CRISPR/Cas9 Gene-Editing platform for solid tumors, provide a solution of two major pain points, GvHD and HvG, aiming to revolutionize cancer. * MT027, the product of target B7H3 received US FDA orphan drug d...
Formosa Pharma and Eyenovia Announce Initiation of Co-Development of Clobetasol Propionate Ophthalmic Suspension, 0.05%, for the treatment of Acute Dry Eye Disease in United States
TAIPEI, Aug. 7, 2024 /PRNewswire/ -- Taiwan-based Formosa Pharmaceuticals ("Formosa", 6838.TWO) announced today that the company has signed a non-binding terms agreement with Eyenovia, Inc. ("Eyenovia", NASDAQ: EYEN), whereby the companies will co-develop Clobetasol Propionate Ophthalmic Suspensi...
I-Mab Appoints U.S. Auditor, PricewaterhouseCoopers LLP (PwC)
Engagement is part of I-Mab's commitment to transition to a U.S.-based biotech PwC to serve as independent registered public accounting firm for FY 2024 ROCKVILLE, Md., Aug. 7, 2024 /PRNewswire/ -- I-Mab (NASDAQ: IMAB) ("I-Mab", the "Company"), a U.S.-based, global biotech company, exclusively f...
Senhwa Biosciences receives US FDA Study May Proceed letter for the Phase I/II study of Silmitasertib (CX-4945) in combination with chemotherapy in children and young adults with relapsed refractory solid tumors
TAIPEI and SAN DIEGO, Aug. 6, 2024 /PRNewswire/ -- Senhwa Biosciences, Inc. (TPEx: 6492), a drug development company focusing on first-in-class therapeutics for oncology, rare diseases, and infectious diseases, today announced receipt of a "Study May Proceed" letter from the U.S. Food and Drug A...
Samsung Biologics joins the Pharmaceutical Supply Chain Initiative as Supplier Partner
* Samsung Biologics to embed PSCI principles into business practices for responsible value chain management * Supplier Partnership reaffirms company's commitment to decarbonize and build resilient supply chains INCHEON, South Korea, Aug. 6, 2024 /PRNewswire/ -- Samsung Biologics (KRX: 207940....
First Patient Enrolled in the US Phase 2 Combination Therapy of Akeso's Ligufalimab with Azacitidine for Myelodysplastic Syndrome
HONG KONG, Aug. 6, 2024 /PRNewswire/ -- Akeso has announced the completion of the first patient enrollment in the US for the phase II clinical trial of its innovative CD47 monoclonal antibody, ligufalimab (AK117), in combination with azacitidine for patients with newly diagnosed higher-risk myelo...
META Pharmaceuticals announces FDA Grants Rare Pediatric Disease Designation to META-001-PH for the Treatment of Primary Hyperoxaluria
HONG KONG, Aug. 5, 2024 /PRNewswire/ -- META Pharmaceuticals Inc. announced that the U.S. Food and Drug Administration (FDA) has granted Rare Pediatric Disease Designation (RPDD) to its investigational new drug META-001-PH for the treatment of primary hyperoxaluria (PH).Primary hyperoxaluria is a...
Antennova Announces CD73 Small Molecule Inhibitor Accepted for Mini Oral Presentation at ESMO Congress 2024
BOSTON, Aug. 5, 2024 /PRNewswire/ -- Antennova, a clinical-stage biotech company focused on oncology today announced that the orally administered CD73 small molecule inhibitor ATN-037(also known as ATG-037) has been accepted for Mini Oral presentation at the 2024 European Society of Medical Oncol...
QBIOTICS WELCOMES STEPHEN DOYLE AS CHIEF EXECUTIVE OFFICER
* Stephen Doyle has more than 24 years of experience in the global pharmaceutical industry, including leadership positions with companies such as Sanofi Aventis and Boehringer Ingelheim. He was most recently Chief Business Officer at Aslan Pharmaceuticals. * Mr Doyle brings extensive knowledg...
Formosa Pharmaceuticals Announces Licensing Agreement with Apotex Inc., for Commercialization of Clobetasol Propionate Ophthalmic Suspension for Ocular Surgery Relief and Recovery
TAIPEI, Aug. 5, 2024 /PRNewswire/ -- Taiwan-based Formosa Pharmaceuticals (" Formosa", 6838.TWO) announced today that the company has entered into an exclusive licensing agreement with Apotex Inc. ("Apotex"), for exclusive rights inCanada to the commercialization of clobetasol propionate ophthalmi...
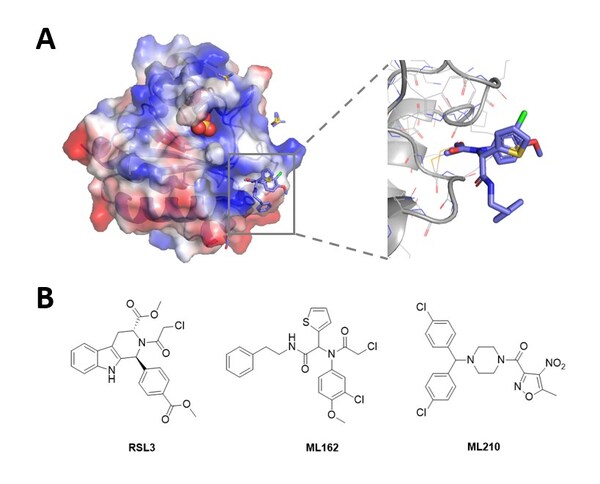
Novel Non-Covalent Hits Against GPX4 Identified Using the RiDYMO® Reinforced Dynamics Platform of DP Technology
Research on ferroptosis is gaining momentum, but the development of small molecule inhibitors faces numerous challenges BEIJING, Aug. 1, 2024 /PRNewswire/ -- Glutathione peroxidase 4 (GPX4) is recognized as a critical regulator of ferroptosis, playing a significant role in lipid and amino acid m...
WuXi Biologics' Four Manufacturing Facilities and Biosafety Testing Center Certified Again by European Medicines Agency for Ten Biologics
WUXI and SUZHOU, China, Aug. 1, 2024 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a leading global Contract Research, Development and Manufacturing Organization (CRDMO), today announced that four manufacturing facilities and Suzhou Biosafety Testing Center inChina have received Good Ma...
Medicilon and Hengrui Pharma Deepen Strategic Collaboration to Support Innovation in ADCs, Small Nucleic Acids, and CGT Drugs
BOSTON, July 31, 2024 /PRNewswire/ -- Recently, Shanghai Medicilon Inc. ("Medicilon") and Jiangsu Hengrui Pharmaceuticals Co., Ltd. ("Hengrui Pharma") reached a strategic collaboration agreement.The cooperative efforts will focus on preclinical evaluation of new drug modalities, particularly ADCs...
JelloX Biotech collaborates with Mayo Clinic to develop AI enhanced 3D pathology imaging technology
HSINCHU, July 31, 2024 /PRNewswire/ -- JelloX Biotech Inc. ('JelloX') is pleased to announce that it has entered into a collaboration through a know-how agreement with Mayo Clinic to further develop and validate their 3D digital imaging and AI analysis technology. JelloX previously participated i...
FDA Grants Orphan Drug Designation (ODD) Status to Zymedi's ZMA001 for Pulmonary Arterial Hypertension
INCHEON, South Korea, July 30, 2024 /PRNewswire/ -- Zymedi (CEO Sunghoon Kim) announced that its first-in-class antibody treatment ZMA001, currently in development for pulmonary arterial hypertension (PAH), has been designated as an Orphan Drug by the U.S. Food and Drug Administration (FDA). Pul...
Porton Advanced and Geneseed Biotech Enter into Strategic Collaboration to Focus on Advancing circRNA Innovative Therapeutics
SUZHOU, China, July 30, 2024 /PRNewswire/ -- On July 29, 2024, Porton Advanced Solutions ("Porton Advanced") announced that it had reached a strategic cooperation with Guangzhou Geneseed Biotech Co., Ltd ("Geneseed Biotech"). Both sides will combine their respective strengths in the field of gene...
Bridge Biotherapeutics Announces Completion of Enrollment in the Phase 2a Clinical Study of BBT-877 for the Treatment of Idiopathic Pulmonary Fibrosis
SEONGNAM, South Korea, July 29, 2024 /PRNewswire/ -- Bridge Biotherapeutics (KQ288330), a clinical-stage biotech company based inSouth Korea developing novel drugs for fibrosis and cancer, today announced that patient participant enrollment has been completed in the Phase 2 clinical study of BBT-...
Daewoong Pharmaceutical Receives Positive Recommendation from IDMC to Continue Developing its First-in-Class PRS Inhibitor, Bersiporocin
SEOUL, South Korea, July 29, 2024 /PRNewswire/ -- Daewoong Pharmaceutical (Co-CEOsChang-Jae Lee and Seongsoo Park) announced a significant milestone in the development of 'Bersiporocin (DWN12088)', a first-in-class PRS inhibitor for idiopathic pulmonary fibrosis (IPF), has taken a significant ste...
Week's Top Stories
Most Reposted
Edufrienz 99: One of Asia's First Digital Platform Advancing Character Education for Future-Ready, Compassionate Children
[Picked up by 318 media titles]
2026-01-23 11:03Agoda Launches Open-Source API Agent to Simplify MCP Server Integrations
[Picked up by 308 media titles]
2026-01-27 13:00UOB prices AUD2 billion in five-year senior unsecured notes
[Picked up by 306 media titles]
2026-01-23 09:00Government of Telangana and Blaize Sign MoU at Davos to Launch Telangana AI Innovation Hub and Advance Applied AI Initiatives
[Picked up by 297 media titles]
2026-01-22 15:16Nokia Strengthens Edge AI Capabilities Through Strategic Collaboration with Blaize on Hybrid Inference Solutions Across Asia Pacific Regions
[Picked up by 292 media titles]
2026-01-27 18:00