Pharmaceuticals
Kangpu Completed Bridging Clinical Study of KPG-818 in China
HEFEI, China, Sept. 20, 2024 /PRNewswire/ -- Kangpu Biopharmaceuticals, a clinical-stage company based inHefei, China, announced today that the Company has successfully completed a bridging clinical study of KPG-818 in healthy subjects inChina. The randomized, double-blind, placebo-controlled,...
CD (Suzhou) Biopharma Announces FDA Clearance for Phase I Clinical Trial of CD-001
SUZHOU, China, Sept. 20, 2024 /PRNewswire/ -- CD (Suzhou) Biopharma
Biosyngen Presents Pioneering"Conditional Activation + Armor Enhancement" SUPER-T technology at ESMO 2024
BARCELONA, Spain, Sept. 19, 2024 /PRNewswire/ -- The highly anticipated 2024 European Society for Medical Oncology (ESMO) Annual Congress has taken place fromSeptember 13 to 17 in Barcelona, Spain. As one of the most influential annual gatherings in oncology, this congress brings together leading...
Best Poster of ESMO 2024! Abbisko Announces Updated Clinical Data of Irpagratinib in HCC
SHANGHAI, Sept. 18, 2024 /PRNewswire/ -- Abbisko Therapeutics (HKEX: 02256) is excited to announce the receipt of the ESMO 2024 Best Poster Award on September 16, 2024. The award was received for the presentation titled "Updated Safety and Efficacy of Irpagratinib (ABSK011) in advanced hepatocell...
Insilico Medicine Reports Positive Phase IIa Results for ISM001-055, a Novel First-in-Class Drug Treatment for Idiopathic Pulmonary Fibrosis (IPF) Designed Using Generative AI
* ISM001-055 is a novel drug designed in-house using generative AI to target TNIK (Traf2- and NCK- interacting kinase) and has progressed through Phase IIa clinical testing. * Preliminary results from this 12-week study demonstrated that ISM001-055 possesses a favorable safety profile and dos...
Bloomage 2024 Mid-Year Report: Driving Growth Through Strategic Transformation to a Solutions Provider
PARSIPPANY, N.J., Sept. 18, 2024 /PRNewswire/ -- On August 23, 2024, Bloomage released its mid-year financial report, reporting a revenue ofRMB 2.811 billion (~USD 385 million) for the first half of the year. This success reflects the company's strategic transformation from a traditional raw mate...
Biosyngen's first-in-class CAR-T asset targeting solid tumors has entered pivotal phase II trial, Phase I trial data debut at ESMO 2024 Annual Congress
BARCELONA, Spain, Sept. 18, 2024 /PRNewswire/ -- The highly anticipated 2024 European Society for Medical Oncology (ESMO) Annual Congress has taken place in Barcelona, Spain. As one of the most influential annual gatherings in oncology, this congress brings together leading cancer experts and rese...
Senhwa Biosciences Announces IND Submission to US FDA for Pilot Study of Pidnarulex Pharmacodynamics in Patients with Advanced Solid Tumors sponsored by NCI
TAIPEI and SAN DIEGO, Sept. 17, 2024 /PRNewswire/ -- Senhwa Biosciences, Inc. (TPEx: 6492), , a drug development company focusing on first-in-class therapeutics for oncology, rare diseases, and infectious diseases, today announced that an Investigational New Drug (IND) application for Pidnarulex ...
Subgroup Analysis from Pivotal WU-KONG1B Study Exhibits Robust Efficacy of Sunvozertinib in Non-Small Cell Lung Cancer Patients with EGFR Exon 20 Insertion Mutations Across Different Baseline Characteristics
Results of subgroup analysis from the pivotal WU-KONG1B study in relapsed or refractory NSCLC with EGFR exon20ins presented at ESMO 2024 * Sunvozertinib demonstrated promising anti-tumor efficacy, regardless of EGFR exon20ins region classification, race, region, baseline brain metastasis, prio...
WestGene Biopharma Presents Groundbreaking mRNA Vaccine Data at ESMO 2024
BARCELONA, Spain, Sept. 16, 2024 /PRNewswire/ -- WestGene Biopharma, a leading innovator in mRNA therapeutics, presented the latest clinical data for its EBV-positive tumour mRNA vaccine, WGc-043, during a mini-oral presentation at the European Society for Medical Oncology (ESMO) Congress 2024. ...
Vazyme Leads Life Science Innovation, Boosting Research Breakthroughs in mIDH1 Cancer Treatment
NANJING, China, Sept. 16, 2024 /PRNewswire/ -- Vazyme (688105.SH), a leading life science technology company, significantly contributed to groundbreaking research published in Science Magazine through its innovative products. The study, which focuses on how mutant IDH1 inhibition activates tumor ...
Antennova Releases Latest Data of CD73 Inhibitor ATN-037, including a DCR of 89.5%, in a Mini Oral at ESMO Congress 2024
* In patients with non-small cell lung cancer (NSCLC) or melanoma who had acquired resistance to checkpoint inhibitors (CPIs), ATN-037 in combination with KEYTRUDA®(pembrolizumab) demonstrated an overall response rate (ORR) of 21.1% and a disease control rate (DCR) of 89.5%. * Data from the...
Breakthrough Therapy designation for Sanbexin sublingual tablets granted by the United States Food and Drug Administration
NANJING, China, Sept. 14, 2024 /PRNewswire/ -- On September 2, 2024, Simcere Pharmaceuticals Group Ltd. (2096.HK) announced that Sanbexin Sublingual Tablets (Edaravone and Dexborneol sublingual tablets), an innovative drug for stroke, has been granted the Breakthrough Therapy designation by the U...
Harbour BioMed Announces the Latest Clinical Data on the First-in-Class Fully Human Anti-B7H7/HHLA2 Monoclonal Antibody HBM1020 at the ESMO Congress 2024
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, Sept. 14, 2024 /PRNewswire/ -- Harbour BioMed (the "Company"; HKEX: 02142), a global biopharmaceutical company committed to the discovery, development, and commercialization of novel antibody therapeutics focusing on oncology and immuno...
MediLink presents YL201 (B7H3 ADC) at ESMO 2024, with over 6-months PFS in SCLC, and showing pan-tumor benefits
* The first publication of clinical data for YL201, featured in an oral presentation at ESMO 2024. * Encouraging antitumor activity of YL201 in multiple solid tumor types, including SCLC, NPC, and wild-type NSCLC, from Phase I escalation and expansion results. * In extensive-stage SCLC pati...
US FDA Grants RPD Designation to Senhwa Biosciences Silmitasertib for Pediatric Neuroblastoma
TAIPEI and SAN DIEGO, Sept. 13, 2024 /PRNewswire/ -- Senhwa Biosciences, Inc. (TPEx: 6492), a new drug development company focusing on first-in-class therapeutics for oncology, rare diseases, and infectious diseases, today announced that its new drug Silmitasertib (CX-4945) was granted a rare pe...
Nona Biosciences Enters into Collaboration Agreement with Umoja Biopharma to Advance In Vivo CAR-T Cell Therapies
CAMBRIDGE, Mass., Sept. 12, 2024 /PRNewswire/ -- Nona Biosciences, a global biotechnology company providing a total solution from "Idea to IND" (I to ITM), announced today that it has entered into a multi-target antibody discovery collaboration with Umoja Biopharma, a transformative immunotherapy...
Stapokibart Was Granted Marketing Approval From National Medical Products Administration For The Treatment Of Moderate-to-severe Atopic Dermatitis in Adults
CHENGDU, China, Sept.12, 2024 /PRNewswire/ -- Keymed Biosciences Inc. (HKEX: 02162) today announced the National Medical Products Administration (the "NMPA") ofChina has recently approved the new drug application for Stapokibart (anti-IL-4Rα monoclonal antibody, trade name:Kangyueda (康悦达), for th...
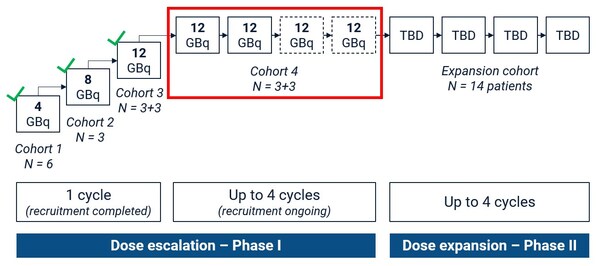
SECuRE trial advances: No dose limiting toxicities and strong preliminary efficacy data in first multi-dose cohort
Highlights * Cohort 4 of the SECuRE trial is the first to assess multiple cycles of 67 Cu-SAR-bisPSMA at the highest dose of 12GBq. * The Safety Review Committee (SRC) assessed early data from the first 3 participants in cohort 4 who received 2 doses of67Cu-SAR-bisPSMA. Two of these participa...
E-Home Household Services Holdings Limited's subsidiary Zhongrun Pharmaceutical and New Zealand's NBL Pharmaceuticals Sign Cooperation Agreement to Expand International Markets
FUZHOU, China, Sept. 12, 2024 /PRNewswire/ -- E-Home Household Services Holdings Limited (NASDAQ:EJH) (the "Company" or "eHome"), an integrated home services provider inChina, announced today that after the detailed business and market due diligence conducted by the Company and the detailed busin...
Week's Top Stories
Most Reposted
Visa partners with Laufey to spread the magic of travel in Asia Pacific; to be Official Payment Partner for Laufey: A Matter of Time Tour
[Picked up by 309 media titles]
2026-03-04 12:35SMU and Fudan Launch Region's First Tech-Focused DBA
[Picked up by 300 media titles]
2026-03-02 09:15Klook's Spring Readiness Index shows how Asia's travelers are preparing for spring travel across Japan, South Korea, and Mainland China
[Picked up by 291 media titles]
2026-03-03 15:49Infobip is set to launch AgentOS to orchestrate autonomous AI-driven customer journeys at scale
[Picked up by 290 media titles]
2026-03-02 09:00COL and NASDAQ-Listed BeLive Holdings Unveil World's First "Microdrama in a Box" in Headline Hong Kong FILMART 2026 Launch
[Picked up by 287 media titles]
2026-03-05 17:14