Pharmaceuticals
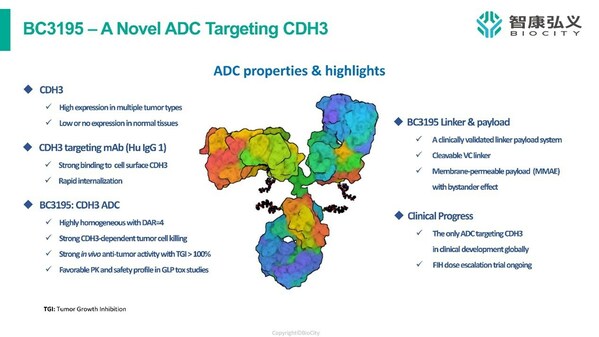
BioCity announces the first patient dosed with its first-in-class CDH3-targeting ADC BC3195 in a Phase 1 Trial
WUXI, China, July 19, 2023 /PRNewswire/ -- BioCity Biopharma, a clinical-stage biopharmaceutical company committed to developing novel and highly differentiated, modality-independent therapeutics for cancer and autoimmune disorders, today announced the dosing of the first patient in a Phase 1a/1b...
Biotheus Announces Strategic Research Collaboration and Worldwide License Agreement with BioNTech
ZHUHAI, China, July 19, 2023 /PRNewswire/ -- Biotheus Inc. ("Biotheus"), a clinical-stage biotech company dedicated to the discovery and development of biologics for oncology and inflammatory diseases, announced today that it has entered into a strategic research collaboration, option and worldwi...
Yikaida CAR-T Cell Therapy Approved as a Second-line Therapy for New Indication
SHANGHAI , July 18, 2023 /PRNewswire/ -- The product of Fosun Kite Biotechnology Co., Ltd., jointly established by Shanghai Fosun Pharmaceutical (Group) Co., Ltd. and Kite Pharma ofthe United States (trade name: Yikaida®) for the treatment of adult large B-cell lymphoma (r/r LBCL) ("this new ind...
Mabwell Announces the NMPA Approval of Novel Trop-2 ADC (9MW2921) for IND
SHANGHAI, July 18, 2023 /PRNewswire/ -- Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, announced that its clinical trial application of 9MW2921 for advanced solid tumor was approved by the National Medical Products Administration (NMPA). 9MW2921 is dev...
Samsung Biologics releases 2023 ESG Report, reaffirming its commitment to net-zero progress and corporate social responsibility
INCHEON, South Korea, July 18, 2023 /PRNewswire/ -- Samsung Biologics (KRX: 207940), a global contract development and manufacturing organization (CDMO), today released its 2023 Environmental, Social and Governance (ESG) Report. The annual report outlines the company's progress toward its sustain...
Viva Biotech's Portfolio Company DTx Pharma Reached the Acquisition Agreement with Novartis
* DTx Pharma's Fatty Acid Ligand Conjugated Oligonucleotide (FALCON™) platform facilitates extra-hepatic delivery of siRNA therapeutics * Acquisition includes DTx-1252 with the potential to deliver a transformative medicine to Charcot-Marie-Tooth Disease Type 1A (CMT1A) patients * Acquisitio...
SK Biopharmaceuticals Makes Big Bets to be 'Big Biotech'
SK Biopharmaceuticals CEO Donghoon Lee maps out its medium to long-term strategy at a press conference SK Biopharmaceuticals mobilizes its cutting-edge modality platforms of radiopharmaceutical therapy (RPT), targeted protein degradation (TPD), and cell and gene therapy (CGT) aligning with its v...
Qilian International Holding Group Ltd Receives Nasdaq Notification Regarding Minimum Bid Price Deficiency
JIUQUAN, China, July 17, 2023 /PRNewswire/ -- Qilian International Holding Group Ltd (NASDAQ: QLI) ("Qilian" or the "Company"), aChina-based pharmaceutical and chemical products manufacturer, received notification (the "Notification Letter") from The Nasdaq Stock Market LLC ("Nasdaq"), datedJuly ...
BIORCHESTRA Wins Grand Prize in 2023 Merck Advance Biotech Grant Program
* Recognition of outstanding in-vivo outcomes from a lead RNA program in CNS * Underpinned by proprietary IV-formulated, Brain-targeting Ribonucleic Acid Interference (RNAi) Nanomedicine (BTRiNTM) platform * BIORCHESTRA to receive in-kind products and services from Merck Group CAMBRIDGE, Mas...
GeneQuantum and InxMed have reached a technology licensing collaboration, providing strong support for innovative ADC drug research and development
SUZHOU, China, July 17, 2023 /PRNewswire/ -- On July 14, 2023, GeneQuantum, a leading pioneer committed to innovative bioconjugation technology, and InxMed, a clinical-stage biotechnology company dedicates on developing innovative therapies targeting drug resistance for hard-to-treat solid tumors...
Harbour BioMed Announces 2023 Interim Results Positive Profit Alert with the Enhancement of Value Driven by Innovation
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, July 13, 2023 /PRNewswire/ -- Harbour BioMed (the "Company"; HKEX: 02142), a global biopharmaceutical company committed to the discovery, development, and commercialization of novel antibody therapeutics focusing on oncology and immunol...
Medicilon Appoints Dr. Liu Jian as President of Drug Discovery Division
BOSTON, July 12, 2023 /PRNewswire/ -- Medicilon, a leading one-stop pharmaceutical preclinical R&D service platform CRO, recently appointed Dr.Liu Jian as the President of Drug Discovery Division. Medicilon recognizes the importance of drug discovery as the cornerstone of new drug innovation. Wit...
Doer Biologics Announces License Agreement with BioNTech
HANGZHOU, China, July 10, 2023 /PRNewswire/ -- Zhejiang Doer Biologics Co., Ltd. ("Doer Bio"), a clinical stage biopharmaceutical company developing innovative biotherapeutics for metabolic diseases and cancers, today announced that it has entered into a license agreement with BioNTech SE (Nasdaq...
Race executes global license agreement with City of Hope to access FTO IP
* Race executes worldwide license agreement with City of Hope, one of the largest cancer research and treatment organisations inthe United States. * Under the agreement, Race will exclusively secure rights to a City of Hope patent application and associated know-how identifying that bisantrene...
Crystal Pharmatech Recognized as "Best Partner" by Allorion Therapeutics for Excellent Services
SUZHOU, China, July 7, 2023 /PRNewswire/ -- Crystal Pharmatech, a leading pharmaceutical technology company, has received dual recognition from its esteemed partner, Allorion Therapeutics (Boston, US / Guangzhou, China) who is an innovation-based biotech company aiming at developing novel drug mo...
280 Bio receives IND approval from the FDA for YL-17231
---The Company announces to initiate Phase 1 Clinical Trial with the KRAS inhibitor YL-17231 in US-- SHANGHAI and SOUTH SAN FRANCISCO, Calif. , July 7, 2023 /PRNewswire/ -- 280Bio, Inc. a clinical stage biotechnology company focused on the development of precision oncology medicines, today annou...
Forge Stronger Partnership for Advanced ADC Development! GeneQuantum Healthcare and Aimed Bio collaborate to develop five innovative ADC drugs
SUZHOU, China, July 6, 2023 /PRNewswire/ -- GeneQuantum Healthcare, a leader in the innovative bioconjugation technologies for ADC new drug development, announced the expansion of its partnership with a South Korean biotech company Aimed Bio today. This strategic collaboration aims to jointly dev...
Hexvix®, A Diagnostic Drug for Bladder Cancer of Asieris has Completed the Phase III bridging trial Enrollment
SHANGHAI, July 6, 2023 /PRNewswire/ -- Asieris Pharmaceuticals (688176), a global biopharma company specializing in discovering, developing and commercializing innovative drugs for the treatment of genitourinary tumors and other related diseases, announced the completion of patient enrollment for...
Registrational Pivotal Phase III Study of Olverembatinib for the First-Line Treatment of Patients with Ph+ ALL Approved by the CDE in China
SUZHOU, China and ROCKVILLE, Md., July 5, 2023 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancer, chronic hepatitis B (CHB), and age-related diseases, announced today that the Center for Drug Evaluation (CDE) of the Chi...
Biosion, Inc. Appoints Furqan Ahmed, PharmD as Vice President and Head of Business Development
NEWARK, Del. and NANJING, China, July 5, 2023 /PRNewswire/ -- Biosion, Inc. ("Biosion"), a global, clinical-stage biotechnology company, today announced the appointment ofFurqan Ahmed, PharmD., as the Vice President and Head of Business Development. In this position, Furqan will be responsible fo...
Week's Top Stories
Most Reposted
Never Miss a Message: Agoda's Customer Support Now Travels With You
[Picked up by 328 media titles]
2026-02-24 12:00NextFin Asia: A New Dedicated Fund for the Catapult: Inclusion SE Asia Program to Further Scale Inclusive Finance Fintechs in ASEAN
[Picked up by 311 media titles]
2026-02-23 08:00Amadeus acquires SkyLink to accelerate the deployment of AI in travel
[Picked up by 310 media titles]
2026-02-26 19:57HBX Group and Traveloka expand strategic partnership to deepen APAC supply and accelerate global distribution
[Picked up by 309 media titles]
2026-02-26 09:30Klook and Osaka Convention & Tourism Bureau sign MoU to advance inbound tourism and foster socio-economic development throughout Osaka Prefecture
[Picked up by 302 media titles]
2026-02-24 16:13