Pharmaceuticals
Vatech Announces Distribution Through Henry Schein to Help Oral Health Professionals Enhance Access to Cutting-Edge Imaging Solutions
HWASEONG, South Korea, Aug. 8, 2023 /PRNewswire/ -- Vatech, a global leader in the dental imaging market, has announced that its products will be distributed byHenry Schein, helping to expand the availability of its cutting-edge products for dental clinics inNorth America. Vatech's portfolio o...
I-Mab to Report Mid-Year 2023 Financial Results, Business and Corporate Updates on August 17, 2023
ROCKVILLE, Md. and SHANGHAI, Aug. 8, 2023 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of pioneering immunotherapies, today announced that it will report business and corporate updat...
Harbour BioMed Appoints Dr. Albert R. Collinson as Independent Non-Executive Director
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, Aug. 8, 2023 /PRNewswire/ -- Harbour BioMed (the "Company"; HKEX: 02142), a global biopharmaceutical company committed to the discovery, development, and commercialization of novel antibody therapeutics focusing on oncology and immunolo...
Gracell Biotechnologies Announces Up to $150 Million Private Placement Financing Joined by a Syndicate of Premier Healthcare Investors
* Oversubscribed transaction led by Vivo Capital and joined by leading healthcare investors including Adage Capital Partners LP, Exome Asset Management, Janus Henderson Investors, Logos Capital, OrbiMed, Pivotal Life Sciences, RA Capital Management and TCGX * $100 million financing upfront wi...
Jacobio Pharma Announces Breakthrough Therapy Designation from China CDE for KRAS G12C Inhibitor Glecirasib for the Treatment of Pancreatic Cancer
BEIJING, SHANGHAI and BOSTON, Aug. 7, 2023 /PRNewswire/ -- Jacobio Pharma (1167.HK), a clinical-stage oncology company focusing on undruggable targets, today announced that Jacobio's in-house KRAS G12C inhibitor glecirasib was granted breakthrough therapy designation (BTD) by the Center for Drug ...
DualityBio Expands Global Strategic Partnership with BioNTech to Accelerate Development of a Third Antibody-Drug Conjugate Therapeutics for Solid Tumors
SHANGHAI, Aug. 7, 2023 /PRNewswire/ -- Duality Biologics (Suzhou) Co. Ltd. ("DualityBio"), a clinical-stage biotech company focusing on the discovery and development of next generation antibody-drug conjugate ("ADC") therapeutics to treat patients with cancer and autoimmune diseases, today announ...
BioCity announces FDA clearance of Investigational New Drug application for its first-in-class CD3/EGFR bispecific antibody
WUXI, China, Aug. 7, 2023 /PRNewswire/ -- BioCity Biopharma today announced that the U.S. Food and Drug Administration (FDA) has cleared the company's Investigational New Drug (IND) application for a Phase 1 study of BC3448 (CD3/EGFR Bispecific antibody, BsAb). As a result, BioCity Biopharma will...
The eClinical Medicine of Lancet Published Phase 2 Results of Ivonescimab for the Treatment of NSCLC
HONG KONG, Aug. 7, 2023 /PRNewswire/ -- Akeso Inc. ("Akeso", the "Company"; 9926.HK), a commercial-stage biopharmaceutical company focused on developing and commercializing first-in-class and best-in-class innovative medicines globally, announced today that eClinical Medicine(IF:15.1), a sub-j...
Ascentage Pharma Received Clearance from U.S. FDA to Proceed with Global Registrational Phase III Clinical Trial for Lisaftoclax (APG-2575) in Previously Treated Patients with CLL/SLL
ROCKVILLE, Md. and SUZHOU, China, Aug. 6, 2023 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancer, chronic hepatitis B (CHB), and age-related diseases, announced today that lisaftoclax (APG-2575), a novel Bcl-2 inhibitor...
JinMed's Nanobathing Products Receive Multiple Patent Certifications and Sign Directed Procurement Agreement with Shanghai Zhongjin HongKang Medical Technology
CHANGZHOU, China, Aug. 3, 2023 /PRNewswire/ -- Jin Medical International Ltd. ("the Company" or "JinMed") (NASDAQ: ZJYL) which is the world's leading provider of rehabilitation equipment. Currently, the Company's nanobathing products have been granted an invention patent and four utility model pa...
ESMO 2023 | Ascentage Pharma to Present Results from Two Clinical Studies, including One Oral Presentation, at 2023 ESMO Congress
SUZHOU, China, and ROCKVILLE, Md., Aug. 2, 2023 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancer, chronic hepatitis B (CHB), and age-related diseases, announced today that it is going to release results from two clinic...
SK Biopharmaceuticals Assembles 'Board of Prominent Experts' to Boost Growth, Innovation
Renowned oncologist Yung-Jue Bang, M.D., Ph.D., chairs the distinguished 5-member Scientific Advisory Board to further advance SK Biopharmaceuticals as a global big biotech SEOUL, South Korea, Aug. 2, 2023 /PRNewswire/ -- SK Biopharmaceuticals, a global biotech company, announced the formation o...
Arbele announces early safety data for novel CDH17xCD3 bispecific T-cell engager at American Society of Clinical Oncology Breakthrough Meeting
SYDNEY, Aug. 2, 2023 /PRNewswire/ -- Arbele, a clinical-stage biotechnology company developing novel immunotherapies targeted for advanced gastrointestinal cancers, presented early safety data from its first-in-class CDH17xCD3 bispecific T-cell engager antibody, ARB202, at the ASCO Breakthrough c...
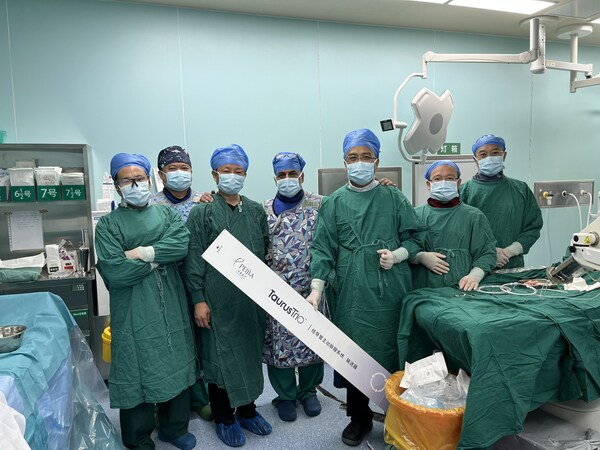
Peijia Medical Announces First Chinese Patient Implanted with TaurusTrio™ Transcatheter Aortic Valve Replacement System
SUZHOU, China, Aug. 1, 2023 /PRNewswire/ -- Peijia Medical (HKG:9996), a leading Chinese domestic player in the high-growth transcatheter valve therapeutic and neurointerventional procedural medical device markets, announced onJuly 31, 2023 that the first Chinese patient of the multi-center ...
Doer Biologics Announces First Subject Dosed in Phase I 12-Week Multiple-Ascending Dose (MAD) Clinical Trial of DR10624 and Received IND Approval From NMPA
HANGZHOU, China, Aug. 1, 2023 /PRNewswire/ -- Zhejiang Doer Biologics Co., Ltd. ("Doer Bio"), a clinical stage biopharmaceutical company developing innovative biotherapeutics for metabolic diseases and cancers, today announces that DR10624, its first-in-class (FIC), tri-specific agonist targeting...
Seegene unveils solutions to popularize molecular diagnostics at 2023 AACC
* Introduced syndromic quantitative PCR assays and a fully automated molecular diagnostic testing system, STARlet AIOS™ * Conducted symposium sessions on the usefulness of PCR testing in diagnosing gastrointestinal diseases * Participated in a presentation event for major Korean in-vitro dia...
Fosun Health Capital reached a cooperation agreement with Medicilon to explore innovative drug research and development together
BOSTON, July 31, 2023 /PRNewswire/ -- On July 25, 2023, Fosun Health Capital established by Fosun Pharma (stock code:600196.SH), reached a strategic partnership with Medicilon (stock code: 688202.SH). Meanwhile StarMab Biology (Suzhou) (jointly invested by the innovation drug research fund of Fo...
111 to Announce Second Quarter 2023 Unaudited Financial Results on August 24, 2023 - Conference Call to Follow
SHANGHAI, July 31, 2023 /PRNewswire/ -- 111, Inc. (NASDAQ: YI) ("111" or the "Company"), a leading tech-enabled healthcare platform company committed to digitally connecting patients with medicine and healthcare services in China, today announced that it will report its unaudited financial result...
Biosion Announces Phase II Study Start for Anti-TSLP mAb BSI-045B in Atopic Dermatitis (ADAMANT)
NEWARK, N.J. and NANJING, China, July 31, 2023 /PRNewswire/ -- Biosion USA, Inc. (Biosion), a global clinical-stage R&D biotechnology company, today announced the phase 2 study initiation for the evaluation of BSI-045B, an anti-TLSP mAb, in the treatment of atopic dermatitis (AD) . "We are pleas...
U.S. FDA Accepts Biologics License Application for GC Biopharma's GC5107B (Immune Globulin Intravenous (Human), 10% Liquid)
YONGIN, South Korea, July 31, 2023 /PRNewswire/ -- GC Biopharma (006280.KS), a global biopharmaceutical company dedicated to specialty plasma-derived therapeutics, today announced that the U.S. Food and Drug Administration (FDA) has accepted the Company's resubmission of the Biologics License App...
Week's Top Stories
Most Reposted
Agoda Launches Agoda Impact Lab at ASEAN Tourism Forum
[Picked up by 322 media titles]
2026-01-29 15:06Wonder Raises USD 12 Million Venture Debt from HSBC Innovation Banking to Drive Growth and Expansion
[Picked up by 322 media titles]
2026-02-02 10:00AI adoption is widespread, but developer confidence is still catching up, Agoda report finds
[Picked up by 312 media titles]
2026-02-03 11:00Colebrook Bosson Saunders Officially Launches Lana, A Circular Ergonomic Laptop Stand for the Hybrid Generation
[Picked up by 303 media titles]
2026-02-03 12:00Mastercard Launches Portfolio of Fleet Solutions in Asia Pacific
[Picked up by 302 media titles]
2026-02-04 09:00