Pharmaceuticals
MGI Announces Commercial Availability of DNBSEQ™ Sequencers* in the United States
SAN JOSE, Calif., June 6, 2022 /PRNewswire/ -- MGI Americas (MGI), today announced that its innovative CoolMPS sequencing chemistry and instruments* will become commercially available inthe United States beginning from August 29,2022. More details about the launch will be revealed at the 22nd ...
Senhwa's Pindnarulex in Combination Study with Pfizer's Talazoparib for the Treatment of Prostate Cancer Granted Approval to Initiate from Australian HREC
TAIPEI and SAN DIEGO, June 6, 2022 /PRNewswire/ -- Senhwa Biosciences, Inc. (TPEx: 6492), a drug development company focusing on first-in-class therapeutics for oncology, rare diseases, and novel coronaviruses, announced that it has received written approval from the Human Research Ethics Committ...
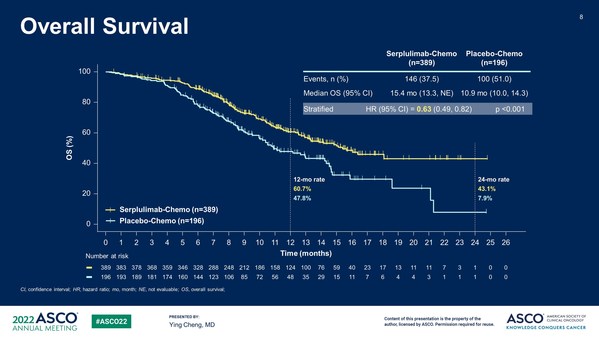
ASTRUM-005: Henlius Released Phase 3 Study Results for the First-line Treatment of Small Cell Lung Cancer of Serplulimab at ASCO 2022
SHANGHAI, June 6, 2022 /PRNewswire/ -- Shanghai Henlius Biotech, Inc. (2696.HK) announced that an international randomized phase 3 study (ASTRUM-005) of HANSIZHUANG (serplulimab), an anti-PD-1 mAb independently developed by Henlius, as first-line treatment for extensive-stage small-cell lung can...
CARsgen Therapeutics Presents Updated Data for CT041 Claudin18.2 CAR T-cells in Solid Tumors at ASCO 2022
SHANGHAI, June 6, 2022 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, announces that at the 2022 American Society of Clinical Oncology (ASCO) Annual M...
CStone and Pfizer announce NMPA approval of sugemalimab in patients with unresectable stage III non-small cell lung cancer
* The National Medical Products Administration approved sugemalimab for the treatment of patients with unresectable stage III non-small cell lung cancer whose disease has not progressed following concurrent or sequential platinum-based chemoradiotherapy * Sugemalimab became the first anti-PD-...
Akeso releases promising data of Ivonescimab (PD-1/VEGF BsAbs, AK112) for advanced NSCLC at ASCO 2022
HONG KONG, June 5, 2022 /PRNewswire/ -- Akeso, Inc. (9926.HK) ( "Akeso" ), a China-based biopharmaceutical company focusing on the development and commercialization of innovative therapeutic antibodies for Oncology & Immunology, released clinical details in poster presentation featuring phase Ib...
Akeso announces oral presentation featuring promising clinical data of Cadonilimab (PD-1/CTLA-4 BsAbs, AK104) for the first-line treatment of R/M cervical cancer at ASCO 2022
HONG KONG, June 5, 2022 /PRNewswire/ -- Akeso, Inc. (9926.HK) ( "Akeso" ), a China-based biopharmaceutical company focusing on the development and commercialization of innovative therapeutic antibodies for Oncology & Immunology, released updated results of Cadonilimab (PD-1/CTLA-4 Bispecific, AK...
KAZIA PRESENTS POSITIVE FINAL DATA FROM PHASE II STUDY OF PAXALISIB IN NEWLY DIAGNOSED GLIOBLASTOMA AT ASCO CONFERENCE
SYDNEY, June 3, 2022 /PRNewswire/ -- Kazia Therapeutics Limited (NASDAQ: KZIA; ASX: KZA), an oncology-focused drug development company, is pleased to announce final data from its phase II study of paxalisib as first line therapy in patients with glioblastoma (NCT03522298). The data is the subj...
Sanyou Super-Trillion Innovative Antibody Drug Discovery Platform Announced - World Premiere
SHANGHAI, June 2, 2022 /PRNewswire/ -- Sanyou Biopharmaceuticals officially
launched STAL (Super-Trillion Antibody Libraries) onJune 3, 2022, which is a
super-trillion innovative antibody drug discovery platform of Sanyou's own
intellectual property.
Alterity Therapeutics Launches ATH434 Phase 2 Clinical Trial for the Treatment of Patients with Multiple System Atrophy
Multiple System Atrophy is a rare, rapidly progressive, neurodegenerative disease that causes profound disability MELBOURNE, Australia, June 2, 2022 /PRNewswire/ -- Alterity Therapeutics (ASX: ATH, NASDAQ: ATHE) ("Alterity" or "the Company"), a biotechnology company dedicated to developing disea...
ProfoundBio Announces Completion of $70 Million Series A+ Financing to Advance Antibody-Drug Conjugate (ADC) Programs into the Clinic
- Advance PRO1184 and PRO1160 into clinical trials - Accelerate multiple programs into preclinical development and further strengthen/expand innovative technology platforms - Build vertically integrated internal capabilities for more efficient drug development WOODINVILLE, Wash. and SUZHOU, China...
First AI to Refine Medical Coding by Exploring Therapeutic Data
SAN FRANCISCO, June 1, 2022 /PRNewswire/ -- Medical AI start-up Aesop
Technology announced a new partnership that made their new product,DxPrime
Standigm Signs MOU with Merck Korea for AI drug Discovery Research
- Merck's retrosynthesis AI software SYNTHIA™ accelerates Standigm's synthesis capability SEOUL, South Korea, June 1, 2022 /PRNewswire/ -- Standigm Inc. ("Standigm"), the leading workflow artificial intelligence (AI) drug discovery company, today announced the signing of a Memorandum of Und...
QureBio Ltd. to Showcase its Q-1802 Clinical Advances at 2022 ASCO Annual Meetings
SHANGHAI, May 31, 2022 /PRNewswire/ -- QureBio Ltd., a clinical-stage biopharmaceutical company focusing on bi-specific antibodies and other engineered bio-therapeutics for the treatment of cancer, inflammation, and other serious disorders, today announced that its Q-1802 clinical program was se...
I-Mab Announces Completion of Patient Enrollment in Phase III Clinical Trial of Eftansomatropin alfa for Treatment of Pediatric Growth Hormone Deficiency
SHANGHAI and GAITHERSBURG, Md., May 31, 2022 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development and commercialization of novel biologics, today announced the completion of patient enrollment in a Phase 3 clinica...
Illuccix® Granted Transitional Pass-Through Payment Status
INDIANAPOLIS, May 31, 2022 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Telix, the Company) today announced that its prostate cancer imaging agent Illuccix® (kit for preparation of gallium Ga 68 gozetotide injection) has been granted Transitional Pass-Through Payment Status by the U.S...
Latest Results from AENEAS Study of Hansoh Pharma's Ameile® Published in Top International Academic Journal JCO
SHANGHAI, May 31, 2022 /PRNewswire/ -- Recently, the Journal of Clinical Oncology (JCO, IF:44.544), an internationally renowned oncology journal, published an online paper on the AENEAS study of Ameile® (Aumolertinib Mesylate Tablets, an innovative drug developed by Hansoh Pharma), which was led ...
AffaMed Therapeutics Announces First Patient Dosed in the US Phase 1 Clinical Trial of AM712 in Retinal Disease
SHANGHAI, May 30, 2022 /PRNewswire/ -- AffaMed Therapeutics ("AffaMed"), a global clinical-stage biopharmaceutical company dedicated to developing and commercializing transformative pharmaceutical, digital and surgical products, announces that the first patient has been dosed in its US Phase 1 st...
Nature Cancer Publishes the Collaborative Study Results by Transcenta and Shanghai Jiao Tong University Scientists
Preclinical Studies on the Potential Application of Transcenta's First-In-Class Gremlin1 Targeting Antibody in the Treatment of Androgen Receptor-Negative/Low Prostate Cancer SUZHOU, China, May 30, 2022 /PRNewswire/ -- Transcenta Holding Limited ("Transcenta") (HKEX: 06628), a clinical stage bio...
InxMed IN10018 at ASCO 2022 demonstrates robust efficacy in patients with platinum-resistant recurrent ovarian cancer
NANJING, China, May 29, 2022 /PRNewswire/ -- InxMed Co., Ltd, a clinical-stage biotechnology company dedicates on developing innovative therapies targeting stroma microenvironment and drug resistance for hard-to-treat solid tumors, is pleased to announce that the clinical data from an open-label...
Week's Top Stories
Most Reposted
Marina Bay precinct partners UOB, Marina Bay Sands and Singapore Tourism Board, together with Disney Cruise Line, to illuminate Singapore's skyline with a fireworks sky show
[Picked up by 328 media titles]
2026-02-19 14:30Never Miss a Message: Agoda's Customer Support Now Travels With You
[Picked up by 326 media titles]
2026-02-24 12:00NextFin Asia: A New Dedicated Fund for the Catapult: Inclusion SE Asia Program to Further Scale Inclusive Finance Fintechs in ASEAN
[Picked up by 311 media titles]
2026-02-23 08:00Klook and Osaka Convention & Tourism Bureau sign MoU to advance inbound tourism and foster socio-economic development throughout Osaka Prefecture
[Picked up by 301 media titles]
2026-02-24 16:13Vitafoods Asia 2026 Expands by 30%: A Bigger, More Dynamic Trade Event with Exciting New Features & Increased International Participation
[Picked up by 288 media titles]
2026-02-23 10:09